��Ŀ����
��ѧ�Ҵӻ��ʳ������ģ�NH4��2SO4�м����ѧʽΪN4H4��SO4��2�����ʣ������ʵľ����к���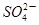 ��
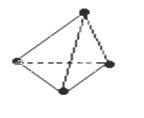
��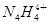 �������ӣ���
�������ӣ���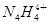 ����������Һʱ��������N4���ӡ�����˵����ȷ����
����������Һʱ��������N4���ӡ�����˵����ȷ����
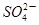 ��
��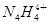 �������ӣ���
�������ӣ���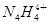 ����������Һʱ��������N4���ӡ�����˵����ȷ����
����������Һʱ��������N4���ӡ�����˵����ȷ����| A��14N��N4��N2��Ϊͬλ�� |
B��N4H4��SO4��2�ĵ��뷽��ʽΪN4H4��SO4��2 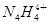 ��2 ��2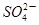 |
| C��N4H4��SO4��2�������ľ�ҡ�K3PO4�Ȼ��ʻ��ʩ�� |
| D��N4H4��SO4��2��ֻ���й��ۼ����������Ӽ� |
C
���������A��ͬ��Ԫ�صIJ�ͬԭ��֮�以��Ϊͬλ�أ�N4��N2Ϊ���ʣ�����B��N4H4��SO4��2�����к���SO42����N4H44���������ӣ�N4H4��SO4��2Ϊ���ӻ�������뷽��ʽΪ��N4H4��SO4��2
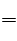 N4H44����2SO42��������C����ľ�ҡ�K3PO4��Ϊǿ�������Σ���ˮ��Һ�Լ��ԣ���N4H44������������Һʱ��������N4���ӣ��ʲ��ܻ��ʹ�ã���ȷ��D��N4H4��SO4��2�������Ӽ�������
N4H44����2SO42��������C����ľ�ҡ�K3PO4��Ϊǿ�������Σ���ˮ��Һ�Լ��ԣ���N4H44������������Һʱ��������N4���ӣ��ʲ��ܻ��ʹ�ã���ȷ��D��N4H4��SO4��2�������Ӽ�������
��ϰ��ϵ�д�
�����Ŀ
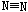 �ų�942KJ����������������Ϣ�������ж�����˵����ȷ���ǣ� ��
�ų�942KJ����������������Ϣ�������ж�����˵����ȷ���ǣ� ��