��Ŀ����
14�������ҹ��ķ��Ԫ�أ��㷺���ڴ���������ҵ��
�ش��������⣺
��1������Ԫ�����ڱ��е�λ��Ϊ��4���ڢ�B�壬��۲�����Ų�ͼΪ
 ��
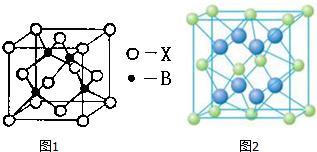
����2������ij��������ľ����ṹ��ͼ1��ʾ��������ʵ��ӵ�е����������Ӹ����ֱ�Ϊ4��2��
��3��V2O5������SO2 ת��ΪSO3�Ĵ�����SO2 ������Sԭ�Ӽ۲���Ӷ�����3�ԣ����ӵ����幹��ΪV�Σ�SO3��̬Ϊ�����ӣ��÷�����Sԭ�ӵ��ӻ��������Ϊsp2��SO3�������廷״�ṹ��ͼ2��ʾ���ýṹ��Sԭ�ӵ��ӻ��������Ϊsp3���ýṹ��S-O���������࣬һ�����Լ140pm����һ�����ԼΪ160pm���϶̵ļ�Ϊa����ͼ2����ĸ�����÷����к���12���Ҽ���
��4��V2O5 �ܽ���NaOH��Һ�У��ɵõ������ƣ�Na3VO4�������������ӵ����幹��Ϊ�������壻Ҳ���Եõ�ƫ�����ƣ��������ӳ���ͼ3��ʾ��������״�ṹ����ƫ�����ƵĻ�ѧʽΪNaVO3��
���� ��1��������֪�����ĺ˵����Ϊ23���������֪����Ԫ�����ڱ��е�λ��Ϊ��4���ڢ�B�壬���ݺ�����ӵĹ�������Ų�˳��֪��1s��2s��2p��3s��3p��4s��3d��4p������ƶ�������Ų�ʽΪ1s22s22p63s23p63d34s2��ע������4s���������3d��������ͣ��������4s����������۲�����Ų�ʽΪ3d34s2���Դ���д�����Ų�ͼ��
��2���ɾ�����֪��Vλ�ڶ�������ģ�O��4��λ�����ģ�2��λ�����ģ�
��3��SO2������Sԭ���γ�2���ļ����µ��Ӷ���Ϊ$\frac{6-2��2}{2}$=1��SO3��̬Ϊ�����ӣ��÷�����Sԭ���γ�3���ļ���û�й¶Ե��ӣ�SO3����������Sԭ���γ�4���ļ����Դ��жϿռ乹�ͺ��ӻ����ͣ�SO3����������ÿ��S�γɣ�����S=O����S-O����S=O�����϶̣�
��4��VO43-�У�V�γ�4���ļ����µ��Ӷ���Ϊ$\frac{5+3-4��2}{2}$=0��Ϊ��������ṹ������״�ṹ��֪ÿ��V��3��O�γ������ӣ���V�Ļ��ϼ�Ϊ+5�ۣ��Դ��ж��γɵĻ�����Ļ�ѧʽ��
��� �⣺��1��������֪�����ĺ˵����Ϊ23���������֪����Ԫ�����ڱ��е�λ��Ϊ��4���ڢ�B�壬���ݺ�����ӵĹ�������Ų�˳��֪��1s��2s��2p��3s��3p��4s��3d��4p������ƶ�������Ų�ʽΪ1s22s22p63s23p63d34s2��ע������4s���������3d��������ͣ��������4s����������۲�����Ų�ʽΪ3d34s2��������Ų�ͼΪ ��
��
�ʴ�Ϊ����4���ڢ�B�壻 ��
��
��2���ɾ�����֪��Vλ�ڶ�������ģ������Ӹ���Ϊ1+8��$\frac{1}{8}$=2��O��4��λ�����ģ�2��λ�����ģ��������Ӹ���Ϊ4��$\frac{1}{2}$+2=4��
�ʴ�Ϊ��4��2��
��3��SO2������Sԭ���γ�2���ļ����µ��Ӷ���Ϊ$\frac{6-2��2}{2}$=1��SO2������Sԭ�Ӽ۲���Ӷ�����3��ΪV�νṹ��
SO3��̬Ϊ�����ӣ��÷�����Sԭ���γ�3���ļ���û�й¶Ե��ӣ���Ϊsp2�ӻ���
SO3����������Sԭ���γ�4���ļ���Ϊsp3�ӻ���SO3����������ÿ��S�γɣ�����S=O����S-O����S=O�����϶̣���a�϶̣��÷����к��ЦҼ���ĿΪ3��4=12��
�ʴ�Ϊ��3��V�Σ�sp2��sp3��a��12��
��4��VO43-�У�V�γ�4���ļ����µ��Ӷ���Ϊ$\frac{5+3-4��2}{2}$=0��Ϊ��������ṹ������״�ṹ��֪ÿ��V��3��O�γ������ӣ���V�Ļ��ϼ�Ϊ+5�ۣ����γɵĻ����ﻯѧʽΪNaVO3��
�ʴ�Ϊ���������壻NaVO3��
���� �����ۺϿ������ʵĽṹ�����ʣ�������ѧ���ķ��������Ŀ��飬ע������ӻ������Լ��۲���������жϣ��Ѷ��еȣ�

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� ̼�����Ļ������ڹ�ũҵ������Ӧ�ù㷺��
̼�����Ļ������ڹ�ũҵ������Ӧ�ù㷺����1����һ�������£�������N2H4����H2O2��Ӧ����N2��H2O����Ӧ�����е������仯��ͼ��ʾ���÷�Ӧ���ڷ��ȷ�Ӧ������д�����ȷ�Ӧ�����ȷ�Ӧ������
�װ���CH3NH2����ˮ��Һ�з�����Ӧ��CH3NH2+H2O?CH3NH2��H2O?CH3CH3++OH-��
CH3NH3Cl��Һ������Ũ�ȵĴ�С˳��Ϊc��Cl-����c��CH3NH3+����c��H+����c��OH-����
��2����ҵ�Ͽɲ���CO��H2�ϳɼ״�����Ӧ����ʽΪ��CO��g��+2H2��g��?CH3OH��g����H��0
T1��ʱ�����ݻ�Ϊ2L�������ܱ������г���CO��H2����ʵ�飬��Ӧ�����в����������±���ʾ��
| ��� | ʵ������ | ��Ӧʱ�� | CO��g��/mol | H2��g��/mol | CH3OH��g��/mol |
| ʵ��� | ���º��� | 0min | 2 | 4 | 0 |
| 10min | 2.8 | ||||
| 20min | 1 | ||||
| ʵ��� | ���Ⱥ��� | 0min | 2 | 4 | 0 |
�ڶ���ʵ��I��20minʱ��Ӧ��ƽ�⣬�����������䣬�����������ٳ���0.1molCO��g����0.2mol CH3OH��g������ƽ�������ƶ�������������������������¶ȱ�ΪT2�棬�ٴδﵽƽ��ʱCH3OH�����ʵ���Ϊ1.2mol����CO��ת����Ϊ60%��
��ʵ��I������ﵽƽ��ʱ��ƽ�ⳣ��KI��K�������������������=������
��3��T��ʱ����̼�ᱵ����Na2SO4��Һ�У���������ת����д����Ӧ�����ӷ���ʽBaCO3��s��+SO42-��aq��=BaSO4��s��+CO32-��aq������Ӧ����ˣ�������Һ�ʼ��ԣ���д�����ԡ��������ԡ������ԡ�����
��1����ͼ1��1mol NO2�����1mol CO���巴Ӧ����CO2�����NO��������������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��NO2��g��+CO��g���TCO2��g��+NO��g����H=-234kJ•mol-1��
��֪��N2 ��g��+2NO2 ��g��?4NO��g����H=+292.3kJ•mol-1��
��Ӧ��2NO��g��+2CO��g��?N2��g��+2CO2��g�� �ġ�H=-760.3kJ•mol-1��
��2��һ���¶��£������Ϊ2L�ĺ����ܱ������г���20mol NO2��5molO2������Ӧ��4NO2��g��+O2��g��?2N2O5��g������֪��ϵ��n��NO2����ʱ��仯���±���
| t��s�� | 0 | 500 | 1000 | 1500 |
| n��NO2����mol�� | 20 | 13.96 | 10.08 | 10.08 |
�ڷ�Ӧ�ﵽƽ���NO2��ת����Ϊ49.6%����Ҫ����NO2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��AD
A�������¶� B�����뺤����ʹ��ϵѹǿ����
C���ٳ���NO2 D���ٳ���4mol NO2��1mol O2
��ͼ2�б�ʾN2O5��Ũ�ȵı仯������C����O2��ʾ��0��500s�ڸ÷�Ӧ��ƽ������v=0.00151mol•L-1•s-1��

| A�� | ���ڸ߷��ӻ����� | |
| B�� | ˮ���ɵõ����ְ����� | |
| C�� | �����ڵ����ʷ�Ӧ�������о� | |
| D�� | ����CuSO4��Һ�����õij�����������ˮ |
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
| A�� | �Ҵ� | B�� | 1-���� | C�� | ���� | D�� | ������ |
| A�� | �ڢܢۢ� | B�� | �ڢۢ� | C�� | �ڢܢۢݢ� | D�� | �ڢݢۢ� |
| Ԫ�� | N | S | O | Si |
| ԭ�Ӱ뾶/10-10m | 0.75 | 1.02 | 0.74 | 1.17 |
| A�� | 0.80��10-10 m | B�� | 1.10��10-10 m | C�� | 1.20��10-10 m | D�� | 0.70��10-10 m |
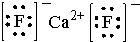 ��������A���Ӿ�������������E����Χ�ɵļ�������״�������壮
��������A���Ӿ�������������E����Χ�ɵļ�������״�������壮