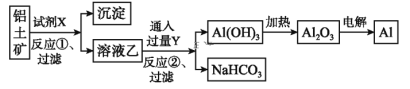
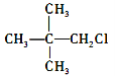
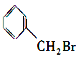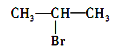��Ŀ����
����Ŀ���������ʵ�����A��B�����2 L���ܱ������У��������з�Ӧ2A(g) + B(g) ![]() xC(g) + 2D(g)����4min���ƽ�⣬���D��Ũ��Ϊ1.0 mol��L��1��c(A) : c(B)��2 : 3����C��ʾ��ƽ��������(C)��0.125 mol��L��1��min��1������˵������ȷ����
xC(g) + 2D(g)����4min���ƽ�⣬���D��Ũ��Ϊ1.0 mol��L��1��c(A) : c(B)��2 : 3����C��ʾ��ƽ��������(C)��0.125 mol��L��1��min��1������˵������ȷ����
A. ��Ӧ������(A)��0.25 mol��L��1��min��1
B. �÷�Ӧ����ʽ�У�x��1
C. 4 minʱ��B�����ʵ���Ϊ2 mol
D. �÷�Ӧ��ƽ�ⳣ��K��1/3
���𰸡�C
��������
������A������B�����ʵ���Ϊamol�����ھ�4min���ƽ�⣬���D��Ũ��Ϊ1.0 mol/L��֪����n(D)= 1.0 mol/L��2L=2.0mol�����ݻ�ѧ��������֪��4minʱ������Cxmol������A2.0mol������B1.0mol��������4minʱ��c(A) : c(B)��![]() ������õ�a=4��������֪������C��ʾ��ƽ�����ʦ�(C)��
������õ�a=4��������֪������C��ʾ��ƽ�����ʦ�(C)��![]() 0.125 mol��L��1��min��1�������֪x=1���÷���ʽΪ��2A(g) + B(g)
0.125 mol��L��1��min��1�������֪x=1���÷���ʽΪ��2A(g) + B(g) ![]() C(g) + 2D(g)��K=
C(g) + 2D(g)��K=![]() =
=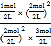 =
=![]() ����������ʽ��ʾΪ��
����������ʽ��ʾΪ��
2A(g) + B(g) ![]() xC(g) + 2D(g)
xC(g) + 2D(g)
��ʼ״̬ amol amol 0 0
4minʱ��Ӧ�� 2.0mol 1mol xmol 2.0mol
4minʱʣ�� 2.0mol 3.0mol 1mol 2mol
A.4minʱ����������A 2.0mol����(A)=![]() ����A��ȷ��
����A��ȷ��
B.������֪������C��ʾ��ƽ�����ʦ�(C)��![]() 0.125 mol��L��1��min��1�������֪x=1����B��ȷ��
0.125 mol��L��1��min��1�������֪x=1����B��ȷ��
C.4 minʱ����������B1mol������������ʣ������B�����ʵ���Ϊ4mol-1mol=3 mol����C����
D.��������ʽ�������ɵõ�K=![]() =
=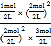 =
=![]() ����D��ȷ��
����D��ȷ��
��ѡC��

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�