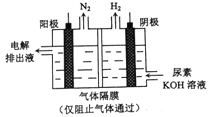
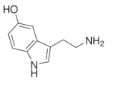
��Ŀ����
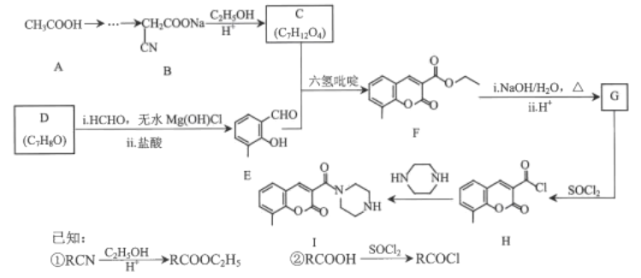
����Ŀ���㶹�ؼ�����������ҽҩ��ũҩ��Ⱦ�Ƶ������й㷺��;��ij�㶹�����������п������������Ķ�����������ԣ���ϳ�·����ͼ��ʾ��
 �ش��������⣺
�ش��������⣺
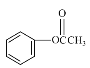
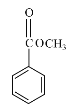
��1��D��������______��F�еĺ��������ŵ�������______��
��2��H��I�ķ�Ӧ����Ϊ______��
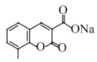
��3��C���Ӿ��жԳƽṹ���û�����Ľṹ��ʽΪ______��
��4��F��G��i�Ļ�ѧ����ʽΪ______��
��5��E��ͬ���칹���У�����������������______�֣�
�ٺ��б���
���ܷ���ˮ�ⷴӦ
���к˴Ź���������4����ҷ����֮��Ϊ3��2��2��1�Ľṹ��ʽΪ______��д��һ�ּ��ɣ���
��6����������·�ߣ�����Ա��ӡ��Ҵ��������ᡢ��ȩΪԭ���Ʊ�![]() �ĺϳ�·��______���������Լ���ѡ����
�ĺϳ�·��______���������Լ���ѡ����
���𰸡��ڼױ��ӻ�2�������� ���� ȡ����Ӧ C2H5COCCH2COOC2H5 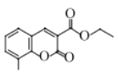 ��NaOH
��NaOH ![]()
 ��CH3CH2OH 6
��CH3CH2OH 6 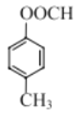 ��
�� ��
��
��������
(1)����D��E�Ľṹ��ʽ�Ա��Լ�D�ķ���ʽ��D�Ľṹ��ʽΪ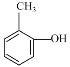 ���ó�D�����ƣ�����F�Ľṹ��ʽ���Ƴ����������ţ�
���ó�D�����ƣ�����F�Ľṹ��ʽ���Ƴ����������ţ�
(2)�Ա�H��I�Ľṹ��ʽ���ó���Ӧ���ͣ�
(3)������Ϣ�٣���C���Ӿ��жԳƽṹ���Ƴ�C�Ľṹ��ʽ��
(4)F�к����������ڼ���Һ�з���ˮ�⣻
(5)����E�Ľṹ����ˮ�⣬˵������������Ȼ����з�����
(1) ����D��E�Ľṹ��ʽ�Ա��Լ�D�ķ���ʽ��D�Ľṹ��ʽΪ ��D������Ϊ�ڼױ��ӻ�2�������ӣ�����F�Ľṹ��ʽ��F�к�����������������
��D������Ϊ�ڼױ��ӻ�2�������ӣ�����F�Ľṹ��ʽ��F�к�����������������
(2)�ԱȽṹ��ʽ��H�еġ�C��Cl�����������ѣ���һ��Ӧ����һ����N��H�����ѣ�����������Cl��H��ϳ�HCl�������ϳ�I���÷�Ӧ����Ϊȡ����Ӧ��
(3)������Ϣ�٣���CΪ�Գƽṹ���Ƴ�C�Ľṹ��ʽΪC2H5COCCH2COOC2H5��
(4)������Ϣ�ڣ��ó�G��Ӧ�����Ȼ�������H�Ľṹ��ʽ��F��Gֻ��1���Ȼ��������ѣ���Ӧ����̼̼˫�����ӵ��Ǹ��������ѣ�����Ӧi�ķ�Ӧ����ʽΪ ��NaOH
��NaOH ![]()
 ��CH3CH2OH��
��CH3CH2OH��
(5)���б������ܷ���ˮ�ⷴӦ������E�Ľṹ��ʽ������Ҫ���ͬ���칹���к���������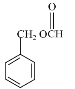 ��
�� ��
��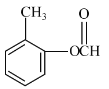 ���ڡ��䡢�����֣���
���ڡ��䡢�����֣��� ������6�֣���4��壬˵����4�ֲ�ͬ����ԭ�ӣ�����ԭ�Ӹ�����Ϊ3��2��2��1����������������
������6�֣���4��壬˵����4�ֲ�ͬ����ԭ�ӣ�����ԭ�Ӹ�����Ϊ3��2��2��1���������������� ��
�� ��
�� ��
��
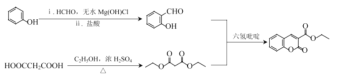
(6)�������̣��Ʊ�![]() ����Ҫ��������
����Ҫ�������� ��C2H5COCCH2COOC2H5����������������D��E������Ʊ�
��C2H5COCCH2COOC2H5����������������D��E������Ʊ� ����Ҫ�ñ����ڼ�ȩ����ˮMg(OH)Cl���Ʊ����Ʊ�C2H5COCCH2COOC2H5����HOOCCH2COOH��C2H5OH��Ũ����������½��У����·����
����Ҫ�ñ����ڼ�ȩ����ˮMg(OH)Cl���Ʊ����Ʊ�C2H5COCCH2COOC2H5����HOOCCH2COOH��C2H5OH��Ũ����������½��У����·���� ��
��