��Ŀ����
����Ŀ�������ת������Դ���úͻ�����������Ҫ�о����⡣��H2S�Ϳ����Ļ������ͨ��FeCl2��CuCl2�Ļ����Һ�з�Ӧ����S��������ת����ͼ��ʾ������˵��������ǣ� ��

A.��ͼʾ��ת���У�Fe3+��CuS���м����
B.��ͼʾ��ת���У����ϼ۲����Ԫ��ֻ��ͭ
C.ͼʾת�����ܷ�Ӧ��2H2S+O2![]() 2S+2H2O
2S+2H2O
D.����1molH2Sת��Ϊ����ʱ����Ҫ����O2�����ʵ���Ϊ0.5mol
���𰸡�B
��������
A���ù����з�����Ӧ��Cu2++H2S��CuS+2H+��CuS+Fe3+��S+Fe2++Cu2+(δ��ƽ)��Fe2++O2��Fe3+(δ��ƽ)���ɴ˿�֪��Fe3+��CuS���м�����A���������⣻
B����ͼ֪�����ϼ۱仯��Ԫ���У�S��Fe��O��Cu��H��Cl�Ļ��ϼ�û�����仯����B�������⣻
C����Aѡ����������������ԭ��Ӧת�Ƶ����غ㡢ԭ���غ��֪���䷴Ӧ���ܷ�ӦΪ��2H2S+O2![]() 2S+2H2O����C���������⣻
2S+2H2O����C���������⣻
D��H2S��Ӧ����S����Ԫ�ػ��ϼ�����2�ۣ�O2��Ӧʱ��Ԫ�ػ��ϼ۽���2������������ԭת�Ƶ����غ��֪������1molH2Sת��Ϊ����ʱ����Ҫ����O2�����ʵ���Ϊ0.5mol����D���������⣻
�ʴ�Ϊ��B��

 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�����Ŀ���ּס�������ѧС�鰲װ������ͼ��ʾ����ͬװ�ã�����̽��Ӱ��H2O2�ֽ����ʵ����ء�

��1������a������___��
��2��MnO2����H2O2�ֽ�Ļ�ѧ����ʽ��___��
��3����С��������ʵ����Ʒ����������������ɱ�����δ��֡�
ʵ���� | ʵ��Ŀ�� | T/K | ���� | Ũ�� |
����ʵ��� | ��ʵ����� | 298 | 3��FeCl3��Һ | 10mL2%H2O2 |
����ʵ��� | ��__ | 298 | ��__ | 10mL5%H2O2 |
��4���ס�����С��ó���ͼ���ݡ�
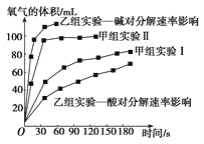
���ɼ���ʵ�����ݿɵó����ֽ���__��
���������о����ᡢ���H2O2�ֽ�Ӱ�����ص�������ͬ�����£�Na2O2��K2O2����ˮ�ų��������ʽϿ����__���������������BaO2������H2SO4��Һ��Ӧ��H2O2���仯ѧ��Ӧ����ʽΪ_��֧����һ������������_��