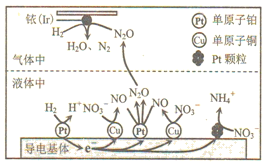
��Ŀ����
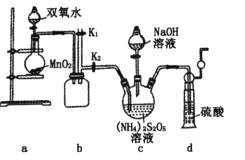
����Ŀ��NiCO3�����ڴ�������ơ��մɵȹ�ҵ������ij������Ʒ���(��Cu��Zn��Fe��Cr������)��ȡNiCO3�Ĺ�����ͼ��ʾ��

��1�������е��Լ�X��ij���Σ��Ļ�ѧʽ��___________��
��2����������ʱ�豣����Һ��40�����ң���6%��H2O2��Һ�����������¶Ȳ�����40����ԭ����______���û�ѧ����ʽ��ʾ����
��3��Fe2+Ҳ������NaClO3���������ɵ�Fe3+�ڽ�СpH������ˮ�⣬�����γɻ�������[Na2Fe6(SO4)4(OH)12]����������ȥ����ͼ��pH���¶ȹ�ϵͼ��ͼ����Ӱ����Ϊ���������ȶ����ڵ���������˵������ȷ����________(����ĸ)��

a����������[Na2Fe6(SO4)4(OH)12]����Ϊ+2��
b��pH���ͻ���߾����������ɻ�����������ԭ����ͬ
c��������������Fe2+ʱ��1 mol NaClO3�õ��ĵ�����Ϊ6NA
d����ҵ�������¶ȳ�������85��95 ��������Na2SO4�����ɻ�����������ʱ��Һ��pHԼΪ1.2��1.8��
��4������Na2CO3��Һʱ��ȷ��Ni2+�Ѿ���ȫ������ʵ�鷽����_______________��
��5��ijС������NiCO3��ȡ�����ص��������ϼ�ʽ������(NiOOH)��������ͼ��
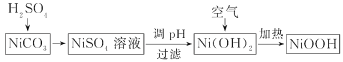
����֪ 25��ʱ��Ksp[Ni(OH)2]��2��10-15�������� pH��9 ʱ����Һ�в�����c(Ni2+)________mol/L��
��д���ڿ����м���Ni(OH)2��ȡNiOOH�Ļ�ѧ����ʽ________________��
�������ص��ҺΪ30%��KOH������ΪMH�����������M������ԭ�ӣ������ʱҲ��ʵ��Ni(OH)2ת��ΪNiOOH����д���ŵ�ʱ�õ�ص��ܷ�Ӧʽ______________��
���𰸡�Na2S 2H2O2![]() 2H2O+O2�� ab ���ã�ȡ�����ϲ���Һ�����μ�1��2��Na2CO3��Һ���������� ��2��10-5 4Ni(OH)2+O2
2H2O+O2�� ab ���ã�ȡ�����ϲ���Һ�����μ�1��2��Na2CO3��Һ���������� ��2��10-5 4Ni(OH)2+O2![]() 4NiOOH+2H2O NiOOH+MH=M+ Ni(OH)2
4NiOOH+2H2O NiOOH+MH=M+ Ni(OH)2
��������
��������ͼ��ij������Ʒ���(��Cu��Zn��Fe��Cr������)�ù��������ܽ⣬���еĽ���ת��Ϊ���������ӣ��ټ�������X�Լ�����ͭ���Ӻ�п����ת��Ϊ���������ȥ����Һ�к��������������Ƚ������ӣ�����(2)��֪��������Һ��40�����ң���6%��H2O2����Fe2+������95������NaOH����pH����ȥ�������ڹ��˺����Һ�ü���̼���Ƶõ�NiCO3���ݴ˷������
(1)��������ͼ�������Լ�X����������ͭ����п����������Լ�X(ij����)ΪNa2S���ʴ�Ϊ��Na2S��
(2)���ڹ��������������ֽ⣬�����������ʱ�豣����Һ��40�����ң��¶Ȳ�����40�����ʴ�Ϊ��2H2O2![]() 2H2O+O2����
2H2O+O2����
(3)a����������[Na2Fe6(SO4)4(OH)12]����Ϊ+1�ۣ������Ϊ-2�ۣ�������Ϊ-1�ۣ������������ϼ۵Ĵ�����Ϊ0����Ϊ+3�ۣ���a����b��pH���ͣ�[Na2Fe6(SO4)4(OH)12]�ܹ������ܽ⣻pH���ߣ�����������ˮ����������������FeOOH�����������������ɻ���������ԭ����ͬ����b����c��������������������������Fe2+���ӷ���ʽΪ6Fe2++ClO3-+6H+ �T6Fe3++Cl-+3H2O��1 mol NaClO3�õ��ĵ�����Ϊ6NA����c��ȷ��d����ͼ���֪�����ɻ�����������Ҫ�ĺ����¶�Ϊ85����95����pHԼΪ1.2��1.8����d��ȷ���������ab���ʴ�Ϊ��ab��
(4)ȷ��Ni2+�Ѿ���ȫ������ʵ�鷽����ȡ�ϲ���Һ������̼������Һ�۲��Ƿ��г������ɣ��ж��������Ƿ�ȫ�������������������Ϊ�����ã����ϲ���Һ�м����μ�1��2��Na2CO3��Һ���������ɣ��ʴ�Ϊ�����ã�ȡ�����ϲ���Һ�����μ�1��2��Na2CO3��Һ���������ɣ�
(5)����֪Ksp[Ni(OH)2]=2��10-15�������� pH��9 ʱ��c(H+)=10-9mol/L����c(OH-)=![]() =10-5mol/L��c(Ni2+)=
=10-5mol/L��c(Ni2+)=![]() =
=![]() =2��10-5 molL-1���ʴ�Ϊ����2��10-5��
=2��10-5 molL-1���ʴ�Ϊ����2��10-5��
�ڿ����м���Ni(OH)2��Ni(OH)2������е�������Ӧ����NiOOH��ˮ����Ӧ�Ļ�ѧ����ʽΪ��4Ni(OH)2+O2![]() 4NiOOH+2H2O���ʴ�Ϊ��4Ni(OH)2+O2
4NiOOH+2H2O���ʴ�Ϊ��4Ni(OH)2+O2![]() 4NiOOH+2H2O��
4NiOOH+2H2O��
�������ص��ҺΪ30%��KOH������ΪMH(���������M������ԭ��)�����ʱҲ��ʵ��Ni(OH)2ת��ΪNiOOH����ŵ�ʱNiOOH������������ԭ��Ӧ����Ni(OH)2����ص��ܷ�ӦΪMH��Ni(OH)2��Ӧ����NiOOH��M����Ӧ����ʽΪNiOOH+MH=M+ Ni(OH)2���ʴ�Ϊ��NiOOH+MH=M+ Ni(OH)2��

 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�����Ŀ���ҹ����������պ��졢�ֻ�����ȶ��Ѿ���������һ��ˮƽ���������в��ϵ�Ӧ�ûش����⡣
Ӧ�� | �����˺š��������� | ��������ˮ½���ܷɻ��㲿�� | ��Ϊ�۵��ֻ���Ļ |
�õ��IJ��� | ���ٸ� | �ѺϽ����Ͻ� | �����ǰ����� |
��1�����������������л��ϳɲ��ϵ���______�����ڽ������ϵ���______��дһ�ּ��ɣ���
��2���ɻ�������ѺϽ����Ͻ���ŵ���______��
��3���ڶԸ����ֹ��еķ�϶���к���ʱ�����������������ڸ��������·�Ӧ��������״̬�µ�������һ�������д���÷�Ӧ�Ļ�ѧ����ʽΪ______����Ӧ����������______��