ĚâÄżÄÚČÝ
ˇľĚâÄżˇżŁ¨1Ł©ŇŃÖŞ˛ÝËᣨH2C2O4Ł©żÉĘąH2SO4ËữµÄKMnO4ČÜŇşÍĘÉ«Ł¬Çë»Ř´đŇÔĎÂÎĘĚ⣺
˘ŮÇ벹ȫ˛˘ĹäĆ˝¸Ă·´Ó¦·˝łĚĘ˝Łş
_____H2C2O4+___KMnO4+___H2SO4 ˇú ___K2SO4+____ MnSO4+___ CO2ˇü + ___H2O
˘ÚÔڸ÷´Ó¦»ąÔ˛úÎďÎŞ________Ł¨Đ´»ŻŃ§Ę˝Ł©
Ł¨2Ł©ÄłČÜŇşÖĐżÉÄÜş¬ÓĐŇÔĎÂŔë×ÓŁşNa+ˇ˘K+ˇ˘Fe3+ˇ˘CO32Łˇ˘SO42Łˇ˘ClŁŁ¬ĎÖ˝řĐĐČçĎÂʵŃ飺
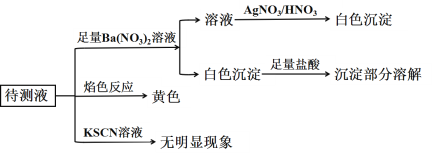
¸ůľÝʵŃéĎÖĎó»Ř´đŁş
¸ĂČÜŇşÖĐŇ»¶¨ş¬ÓеÄŔë×ÓĘÇ______________Ł»Ň»¶¨˛»ş¬ÓеÄŔë×ÓĘÇ____________Ł»ÎŢ·¨Č·¶¨µÄŔë×ÓĘÇ____________________ˇŁ
ˇľ´đ°¸ˇż5 2 3 1 2MnSO4 10 8 MnSO4 Na+ˇ˘CO32Łˇ˘SO42Łˇ˘ClŁ Fe3+ K+
ˇľ˝âÎöˇż
Ł¨1Ł©˘ŮŇŔľÝŃő»Ż»ąÔ·´Ó¦Öеç×ÓµĂʧĘŘşăşÍÔ×ÓĘŘşă˝â´đŁ»
˘ÚŇŔľÝŃő»Ż»ąÔ·´Ó¦ÖĐŃő»ŻĽÁËůş¬ÔŞËŘ»ŻşĎĽŰ˝µµÍ˝â´đŁ»
Ł¨2Ł©¸ůľÝʵŃéĎÖĎó˝áşĎŔë×ÓµÄĐÔÖĘ·ÖÎö˝â´đˇŁ
Ł¨1Ł©˘Ů¸Ă·´Ó¦ÖĐMnÔŞËŘ»ŻşĎĽŰÓÉ+7ĽŰ±äÎŞ+2ĽŰˇ˘CÔŞËŘ»ŻşĎĽŰÓÉ+3ĽŰ±äÎŞ+4ĽŰŁ¬ĆäתŇƵç×Ó×ÜĘýÎŞ10Ł¬ËůŇÔ˝áşĎµç×ÓµĂʧĘغ㡢Ô×ÓĘŘşăĹäĆ˝·˝łĚʽΪ5H2C2O4+2KMnO4+3H2SO4Ł˝K2SO4+2MnSO4+10CO2ˇü+8H2OŁ»
˘Ú˛ÝËáÓë¸ßĂĚËáĽŘÔÚËáĐÔ»·ľłĎ·˘Éú·´Ó¦Ł¬¸ßĂĚËáĽŘ×öŃő»ŻĽÁ±»»ąÔŁ¬Ôڸ÷´Ó¦ÖĐ+7ĽŰĂĚ˝µĽŰÎŞ+2ĽŰĂĚŔë×ÓŁ¬ËůŇÔ»ąÔ˛úÎďÎŞMnSO4Ł»
Ł¨2Ł©´ý˛âŇşĽÓČëKSCNČÜŇşŁ¬ÎŢĂ÷ĎÔĎÖĎóŁ¬ÔňČÜŇşÖĐÎŢČýĽŰĚúŔë×ÓŁ¬ŃćÉ«·´Ó¦łĘ»ĆÉ«Ł¬ËµĂ÷ş¬ÓĐÄĆŔë×ÓŁ¬ĽÓČë×ăÁżĎőËá±µČÜŇş˛úÉú°×É«łÁµíŁ¬ĽÓČë×ăÁżŃÎËá°×É«łÁµí˛ż·ÖČÜ˝â˵Ă÷łÁµíÎŞÁňËá±µˇ˘ĚĽËá±µ»ěşĎÎÔňČÜŇşÖĐş¬ÓĐÁňËá¸ůŔë×Óˇ˘ĚĽËá¸ůŔë×ÓŁ¬ĎňĽÓČë×ăÁżĎőËá±µČÜŇşşóąýÂ˵õ˝µÄÂËŇşÖĐĽÓČëĎőËáËữµÄĎőËáŇřČÜŇş˛úÉú°×É«łÁµíÎŞÂČ»ŻŇřłÁµíŁ¬ÔňČÜŇşÖĐŇ»¶¨ş¬ÓĐÂČŔë×ÓŁ¬Í¨ąýŇŃÓĐĘÂʵÎŢ·¨Č·¶¨ĘÇ·ń´ćÔÚĽŘŔë×ÓŁ¬ËůŇÔ¸ĂČÜŇşÖĐŇ»¶¨ş¬ÓеÄŔë×ÓĘÇNa+ˇ˘CO32Łˇ˘SO42Łˇ˘ClŁŁ»Ň»¶¨˛»ş¬ÓеÄŔë×ÓĘÇFe3+Ł»ÎŢ·¨Č·¶¨µÄŔë×ÓĘÇK+ˇŁ

ˇľĚâÄżˇżŁ¨ĚâÎÄŁ©˘ńŁ®»ŻŃ§żÎÉĎŔĎʦŃÝĘľÁËČçĎÂÍĽµÄʵŃ飺
ʵŃéʱŁ¬˝«Á˝ĆřÇňÄÚµÄNaHCO3şÍNa2CO3ͬʱµąČëĘÔąÜÖСŁŇŃÖŞŃÎËáĘÇ×ăÁżµÄŁ¬ÇŇŐűĚ××°ÖĂĆřĂÜĐÔÁĽşĂŁ¬»Ř´đĎÂÁĐÎĘĚ⣺
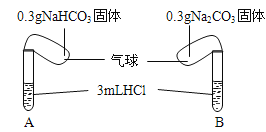
Ł¨1Ł©ĘµŃéąýłĚÖй۲쵽µÄĎÖĎóĘÇŁş________________Ł®
Ł¨2Ł©ĘµŃé˝áĘřşóŁ¬Á˝ĘԹܵÄĆřÇň´óСÓвîŇ죬´óСĆřÇňŁ¨°üş¬ĘԹܣ©Ěĺ»ýÖ®±ČÔĽÎŞŁ¨Ěî×îĽňµĄŐűĘý±ČŁ©__________Ł®
˘ňŁ®µ±Ľ×ͬѧÓĂĘÖ´ĄĂţÉĎĘöĘÔąÜʱŁ¬ŇâÍâ·˘ĎÖAĘԹܱäŔ䣬¶řBĘԹܷ˘ČČŁ¬Ëű°ŃŐâ¸ö·˘ĎÖ¸ćËßÁËͬ×ŔŇŇͬѧŁ¬˛˘Óɴ˵óö˝áÂŰŁşNaHCO3şÍHCl·´Ó¦ÎŞÎüČČ·´Ó¦Ł¬¶řNa2CO3şÍHCl·´Ó¦ÎŞ·ĹČČ·´Ó¦Ł®ŇŇͬѧȴ¶Ô´Ë˝áÂŰČ´łÖ»łŇɵÄ̬¶ČŁ®ÎŞ´ËŁ¬Á˝Î»Í¬Ń§ÔÚżÎÍâ»î¶ŻÖĐĽĚĐř˝řĐĐÁËĎÂÁĐʵŃ飨ÿ´ÎʵŃé¸÷×ö3´ÎĆ˝ĐĐʵŃ飬ȡƽľůÖµŁ©Łş
ĐňşĹ | ĘÔĽÁ1 | ĘÔĽÁ2 | »ěşĎÇ°ÎÂ¶Č | »ěşĎşóÎÂ¶Č |
˘Ů | 35mLË® | 2.5g NaHCO3ąĚĚĺ | 20ˇć | 18.5ˇć |
˘Ú | 35mLË® | 3.2g Na2CO3ąĚĚĺ | 20ˇć | 24.3ˇć |
˘Ű | 35mLϡŃÎËá | ş¬2.5g NaHCO3µÄ±ĄşÍČÜŇş32.5mL | 20ˇć | 19ˇć |
˘Ü | 35mLϡŃÎËá | ş¬3.2g Na2CO3µÄ±ĄşÍČÜŇş23.1mL+10mLË® | 20ˇć | 24.2ˇć |
˘Ý | 35mLϡŃÎËá | 2.5g NaHCO3ąĚĚĺ | 20ˇć | 16.2ˇć |
˘Ţ | 35mLϡŃÎËá | 3.2g Na2CO3ąĚĚĺ | 20ˇć | 25.1ˇć |
ÇëÄă°ďÖúĚîĐ´ĎŕąŘÄÚČÝŁş
Ł¨1Ł©¸ĂŃĐľż±¨¸ćµÄĚâÄżĘǡ¶___________________________ˇ·Ł®
Ł¨2Ł©¸ĂʵŃéÖĐËůÓõÄŇÇĆ÷łýĘԹܡ˘Ň©ł×Ł¨»ňVĐÍÖ˝˛ŰŁ©ˇ˘ĆřÇňˇ˘˛ŁÁ§°ôˇ˘ÉŐ±ˇ˘ÁżÍ˛ˇ˘±ŁÎÂĆżÍ⣬»ąĐčŇŞµÄŇÇĆ÷ĂűłĆĘÇ______________________Ł®
Ł¨3Ł©ĘµŃé˘ŮşÍ˘ÚµÄÄżµÄĘÇ_________________________________________________Ł®
Ł¨4Ł©Í¨ąýÉĎĘöʵŃéżÉµĂłöµÄ˝áÂŰĘÇŁş___________________________________Ł¨Đ´łöÁ˝ĚőĽ´żÉŁ©Ł®
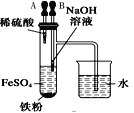
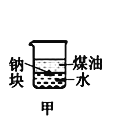
ˇľĚâÄżˇżĎÂÁĐ×°ÖĂËůĘľµÄʵŃéÖĐŁ¬˛»ÄܴﵽʵŃéÄżµÄĘÇ
|
|
|
|
AŁ®ł¤Ę±Ľäż´µ˝Fe(OH)2°×É«łÁµí | BŁ®Ö¤Ă÷¦Ń(ĂşÓÍ)< ¦Ń(ÄĆ) < ¦Ń(Ë®) | CŁ®Ě˝ľżŃő»ŻĐÔŁş KMnO4ŁľCl2ŁľI2 | DŁ®±Č˝ĎNaHCO3ˇ˘Na2CO3µÄČČÎȶ¨ĐÔ |
A. A B. B C. C D. D
ˇľĚâÄżˇżĎÂÁС°ĘµŃé˝áÂۡ±Ó롰ʵŃé˛Ů×÷Ľ°ĎÖĎóˇ±Ďŕ·űµÄŇ»×éĘÇ
ѡĎî | ʵŃé˛Ů×÷Ľ°ĎÖĎó | ʵŃé˝áÂŰ |
A | ĎňÄłČÜŇşÖĐĽÓČë | ¸ĂČÜŇşÖĐŇ»¶¨ş¬ÓĐ |
B | ĎňÄłČÜŇşÖĐĽÓČëϡŃÎËᣬÓĐÎŢÉ«ĆřĚĺ˛úÉú | ¸ĂČÜŇşÖĐŇ»¶¨ş¬ÓĐ |
C | ĎňÄłČÜŇşÖĐĽÓČë | ¸ĂČÜŇşÖĐŇ»¶¨ş¬ÓĐ |
D | ĎňÄłČÜŇşÖĐĽÓČë·ÓĚŞČÜŇşŁ¬ČÜŇş±äÎŞşěÉ« | ¸ĂČÜŇşÖĐŇ»¶¨ş¬ÓĐ |
A. A B. B C. C D. D