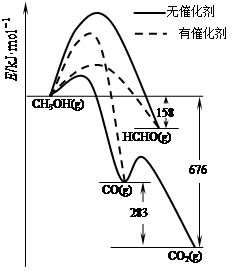
��Ŀ����
���������������������ˮ��ҵ��ˮ����������������������Ͽ졣ʵ���ҿ��ö�������Ϊ��Ҫԭ���Ʊ�������ء��䲿���������£�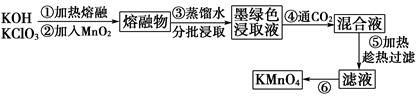
��1���ڢٲ��в��������������ô�������ԭ���ǣ��û�ѧ����ʽ��ʾ��_______________________________________________________________��
��2��KOH��KClO3��MnO2���۷�Ӧ����ī��ɫK2MnO4�Ļ�ѧ����ʽΪ________________________________________________________________��
��3���ڢܲ�ͨ��CO2������ʹMnO42-������Ӧ������MnO4-��MnO2����K2MnO4��ȫ��Ӧʱ��ת��ΪKMnO4�İٷ���ԼΪ____________________����ȷ��0.1%����
��4���ڢݲ����ȹ��˵�Ŀ����________________________________��
��5���ڢ�����Ũ����Һ����ϸС��������ʱ��ֹͣ���ȣ���ȴ�ᾧ��___________��ϴ�ӡ������������У��¶Ȳ��˹��ߣ���Ϊ_________________��
��1�� SiO2��2KOH=K2SiO3��H2O
��2��6KOH��KClO3��3MnO2 KCl��3K2MnO4��3H2O����3��66.7%
KCl��3K2MnO4��3H2O����3��66.7%
��4�����ٹ��˵���ģ����ֹ���¹�����KMnO4��������ģ�
��5�����ˡ��¶ȹ���KMnO4��ֽ�
����

ij̽��С�齫һ�����ӷ���������õ���Cu��Al��Fe������Au��Pt�Ƚ����Ļ�������������Ʊ�����ͭ�������ˮ�Ȼ����ķ�����
��֪��Cu2+ + 4NH3��H2O��[Cu(NH3)4]2+ + 4H2O
��ش��������⣺
��1�������Cu���ᷴӦ�����ӷ���ʽΪ ��
��2������ڼ�H2O2�������� ������2Ϊ(�ѧʽ) ��
��3������ݲ���ֱ�Ӽ�����ˮ�������� ��
��4������Һ1��Cu2+��Ũ��Ϊ0��02mol��L-1����������ͭ��ʼ����ʱ��pH =
(��֪��Ksp[Cu(OH)2]��2��0��10-20)��
��5����֪��2Cu2+��4I-�� 2CuI����I2 I2��2S2O32-�� 2I-��S4O62-
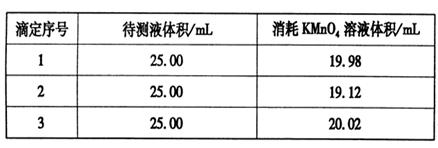
ijͬѧΪ�˲ⶨCuSO4��5H2O��Ʒ�����������ɰ����·�����ȡ3��00g��Ʒ����ˮ�ܽ����������KI��Һ����ַ�Ӧ����ˡ�ϴ�ӣ�����Һϡ����250mL��ȡ50mL���������Һ��ָʾ������0��080 mol��L-1 Na2S2O3����Һ�ζ����ﵽ�ζ��յ�������� ��
�Ĵ�ƽ��ʵ���ȥNa2S2O3����Һ�������£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����Na2S2O3����Һ(mL) | 25��00 | 25��02 | 26��20 | 24��98 |
�˲�Ʒ��CuSO4��5H2O����������Ϊ ��