��Ŀ����
����9�֣�A��B��C��D��E�������ʵ���ɫ������ɫ(����ɫ�ܲ���)��A��B��ˮ��Ӧ��������ų���A��ˮ��Ӧ�ų���������л�ԭ�ԣ�B��ˮ��Ӧ�ų���������������ԣ�ͬʱ��������ҺC.C��������CO2��Ӧ����D���������CO2��Ӧ����E.E����������D.���ƶϣ�
��A��_____________��B��_____________��C��_____________��D��_____________��E��_____________(д��ѧʽ)
�ư�Ҫ��д���йط�Ӧ�Ļ�ѧ����ʽ��
B��ˮ��Ӧ�Ļ�ѧ����ʽ
E��������D�Ļ�ѧ����ʽ
��A��_____________��B��_____________��C��_____________��D��_____________��E��_____________(д��ѧʽ)
�ư�Ҫ��д���йط�Ӧ�Ļ�ѧ����ʽ��
B��ˮ��Ӧ�Ļ�ѧ����ʽ
E��������D�Ļ�ѧ����ʽ
��9�֣���A��K B��K2O2 C��KOH D��K2CO3 E��KHCO3��5�֣�
��2K2O2+2H2O��4KOH+O2�� 2KHCO3 K2CO3+CO2��+H2O��4�֣�
K2CO3+CO2��+H2O��4�֣�
��2K2O2+2H2O��4KOH+O2�� 2KHCO3
 K2CO3+CO2��+H2O��4�֣�
K2CO3+CO2��+H2O��4�֣���

��ϰ��ϵ�д�
�����Ŀ
 ��������ʵ�飺
��������ʵ�飺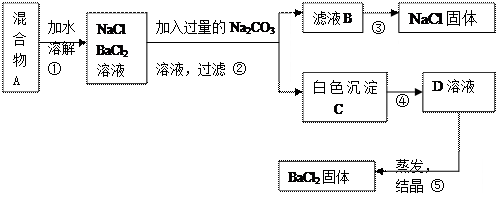
 a2O2��CO2
a2O2��CO2
 +��Mg2+��SO42-���������ӣ�ʵ�����ᴿNaCl���������£�
+��Mg2+��SO42-���������ӣ�ʵ�����ᴿNaCl���������£�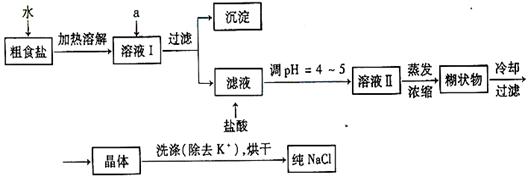 �ṩ���Լ����£�����Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ Ba(NO3)2��Һ
�ṩ���Լ����£�����Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ Ba(NO3)2��Һ 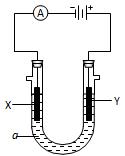
 Cl(Ũ)
Cl(Ũ)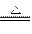 MnCl2��Cl2����2H2O
MnCl2��Cl2����2H2O ������ţ����Ʊ����ռ��������Cl2��װ��
������ţ����Ʊ����ռ��������Cl2��װ��