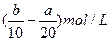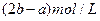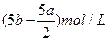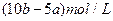��Ŀ����
��9�֣�ijѧ���� ��������ʵ�飺
��������ʵ�飺
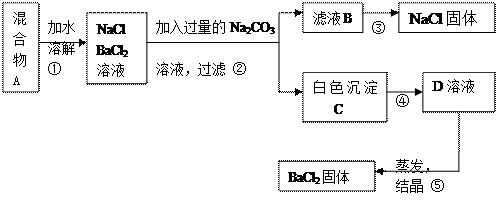
(1)��ͬѧ��ʵ��Ŀ����_____________________________��
(2)��ͼ�۲�������Ϊ________________���ܼ����Լ�Ϊ ��
(3)����ʵ�鷽���õ���NaCl�����п϶�����__________(�ѧʽ)���ʣ�Ϊ�˽����������������˵õ�����Һ�м���������______________��
(4)д������C�Ļ�ѧ����ʽ____________________________��
 ��������ʵ�飺
��������ʵ�飺
(1)��ͬѧ��ʵ��Ŀ����_____________________________��
(2)��ͼ�۲�������Ϊ________________���ܼ����Լ�Ϊ ��
(3)����ʵ�鷽���õ���NaCl�����п϶�����__________(�ѧʽ)���ʣ�Ϊ�˽����������������˵õ�����Һ�м���������______________��
(4)д������C�Ļ�ѧ����ʽ____________________________��
����9��=2+2+2+3��
��1������BaCl2��N aCl���ֹ������������������Ҳ���ԣ�
aCl���ֹ������������������Ҳ���ԣ�
��2���������ᾧ ��������������ϡ����
��3��Na2CO3 �� ϡ����
��4��BaCl2+ Na2CO3��BaCO3��+2NaCl
��1������BaCl2��N
 aCl���ֹ������������������Ҳ���ԣ�
aCl���ֹ������������������Ҳ���ԣ���2���������ᾧ ��������������ϡ����
��3��Na2CO3 �� ϡ����
��4��BaCl2+ Na2CO3��BaCO3��+2NaCl
��

��ϰ��ϵ�д�
�����Ŀ