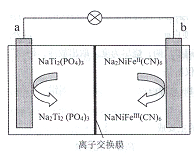
��Ŀ����
����Ŀ���ܼ��仯����������������Ҫ���ã��ش���������
(1)��Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ_________________��δ�ɶԵ�����Ϊ________________��
(2)�����[Co(NH3)4(H2O)2]Cl3������Ҫ������
��H2O�ķе�___ (����ڡ����ڡ�)H2S,ԭ����_______��H2O��O���ӻ���ʽΪ_____��H2O��_____����(����ԡ��Ǽ��ԡ�)��
��[Co(NH3)4(H2O)2]Cl3Co3+��λ��Ϊ___�������ӵ����幹����___________��[Co(NH3)4(H2O)2]Cl3������������NH3���ӱ�Clȡ�������γɵ�[Co(NH3)2(H2O)2] 3+�ļ����칹��������(�����ǹ�ѧ�칹)___________�֡�
(3)����������______���γɵľ��壻CoO��FeO�ľ���ṹ���;����Ȼ��Ƶ���ͬ��Co2+��Fe2+�����Ӱ뾶�ֱ�Ϊ74.5pm��78pm,���۵�CoO______FeO��
(4)һ�����ܵľ�����ͼ������ÿ��Co2+����Χ������ӽ����Ҿ�����ȵ�Co2+����_____������������Co2+��O2-����С����Ϊacm����CoO�ľ����ܶ�Ϊ_______(�ú�NA��a�Ĵ���ʽ��ʾ�����g/cm3,��֪:M(Co)=59g/mol��M(O)=16g/mol���谢��ӵ�����ΪNA)��

���𰸡� 1s22s22p63s23p63d74s2��[Ar]3d74s2 3 ���� H2O���Ӽ���γ���� sp3 ���� 6 ������ 5 ���� �� 12 ![]()
��������(1)��Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d74s2��[Ar]3d74s2��δ�ɶԵ�����Ϊ3����
(2) ��H2O�ķе����H2S��ԭ����H2O���Ӽ���γ������H2O��O���ӻ���ʽΪsp3��ˮ�����Ǽ��Է��������������[Co(NH3)4(H2O)2]Cl3����Co3+��λ��Ϊ6���������ӵ����幹���ǰ����壻��[Co(NH3)4(H2O) 2]Cl3��������NH3���ӱ�Clȡ�������γɵ�[Co(NH3)2(H2O) 2] 3+�ļ����칹�����������֣�
(3) ���������ɽ������γɵľ��壻CoO��FeO�ľ���ṹ���;����Ȼ��Ƶ���ͬ������Co2+�����Ӱ뾶74.5pmС��Fe2+�����Ӱ뾶78pm������CoO���۵����FeO����
(5)��CoO�ľ����ṹ��֪��ÿ��Co2+����Χ������ӽ����Ҿ�����ȵ�Co2+����12������������Co2+��O2-����С����Ϊacm�����þ����ı߳�Ϊ2a cm�������Ϊ8 a3 cm3���þ�������4��CoO������������Ϊ![]() g�������ܶ�Ϊ
g�������ܶ�Ϊ![]() g/cm3��
g/cm3��