��Ŀ����
16��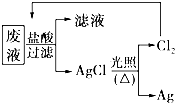 ����Ժǿ������ˮ���������̱����֡���������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�У����д�����Mg2+��Al3+��Cu2+��Ag+���Է����ش��������⣺
����Ժǿ������ˮ���������̱����֡���������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�У����д�����Mg2+��Al3+��Cu2+��Ag+���Է����ش��������⣺��1���÷�Һ�п��ܴ������ڵ�һ����������B��ѡ����ţ���
A��SO42-����B��NO3-C��Cl- ��D��CO32-
��2�������Һ����Ԫ�صĺ������轫��ӷ�ˮ��Ʒ�з�����������õ��Լ�������Al3++4OH-=AlO2-+2H2O
��3��Ϊ�˻��շ�Һ�еĽ�������ijͬѧ��������·����������÷��������108g��Ϊ��֤����Ⱦ������������ѭ�����ã�������Ӧ�ṩ��״���µ�����11.2L��
���� ��1����Mg2+��Al3+��Cu2+��Ag+���κ����Ӷ�����Ӧ�����ӿɴ������棻
��2��������������Ϊ����������������ʽ��з��룻
��3��n��Ag��=$\frac{108g}{108g/mol}$=1mol����Ϸ�Ӧ�Ĺ�ϵʽ2AgCl��Cl2��H2���㣮
��� �⣺��1������SO42-��CO32-��Cl-������Ag+��Ӧ����������ˮ������ˮ�ij�����ֻ��B���ϣ��ʴ�Ϊ��B��
��2����������Ϊ���������������ǿ����룬��ط�Ӧ�����ӷ���ʽΪAl3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al3++4OH-=AlO2-+2H2O��
��3��n��Ag��=$\frac{108g}{108g/mol}$=1mol����Ӧ�Ĺ�ϵʽΪ2AgCl��Cl2��H2����n��H2��=0.5mol��
V��H2��=0.5mol��22.4L/mol=11.2L��
�ʴ�Ϊ��11.2��
���� ���⿼���Ϊ�ۺϣ��漰���ӹ��桢���ӷ���ʽ����д�����㣬Ϊ�߿��������ͣ�������ѧ��Ԫ�ػ�����֪ʶ���ۺ����ã��ѶȲ���ע����������Ϣ�Լ�������ӵ����ʣ�

��ϰ��ϵ�д�
�����Ŀ
6������˵����ȷ���ǣ�������
| A�� | CH4���ӵ����ģ�ͣ� | B�� | �Ҵ��Ľṹʽ�� | ||
| C�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | D�� | ������ļ���ʽ��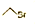 |
4����0.2mol/L NH4Cl��Һ��0.1mol/L NaOH��Һ�������Ϻ���Һ�������������ʵ���Ũ�ȵĹ�ϵ��ȷ���ǣ�������
| A�� | c��NH4+��=c��Na+��=c��OH-����c��NH3•H2O�� | B�� | c��NH4+����=c��Na+����c��NH3•H2O����c��OH-�� | ||
| C�� | c��NH4+������c��Na+����c��OH-����c��NH3•H2O�� | D�� | c��NH4+����c��Na+����c��NH3•H2O����c��OH-�� |
11��Ҫ֤��ij��Һ�в���Fe3+ �����ܺ���Fe2+��Ӧ���е�ʵ����������˳��Ϊ���ټ���������ˮ���ڼ�������KMnO4��Һ���ۼ�������KSCN��Һ��������
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �٢ڢ� |
8�����Ƶ�˼ά�����ڻ�ѧѧϰ���о��г����������Ľ��ۣ�������Ƴ��Ľ�������Ҫ����ʵ���ļ�����ܾ�������ȷ������м������ƽ�������ȷ���ǣ�������
| A�� | ���ˮ��Һ�����ԣ��������Ե�ˮ��ҺҲһ�������ˮ��Һ | |
| B�� | Fe3O4��д��FeO?Fe2O3��Pb3O4Ҳ��д��PbO?Pb2O3 | |
| C�� | �ɵ������MgCl2����ȡ����þ��Ҳ�ܵ������HCl����ȡ���� | |
| D�� | ��2����Ԫ���⻯���ȶ���˳����HF��H2O��NH3�����3����Ԫ���⻯���ȶ���˳����HCl��H2S��PH3 |
5������˵����ȷ���ǣ�������
| A�� | SO2��ʹƷ����Һ��ɫ | B�� | S��O2��ȼ�տ�����SO3 | ||
| C�� | �ó���ʯ��ˮ�ɼ���CO2��SO2 | D�� | ����������ȼ������������ |
 ����
���� ����
����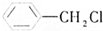 ����
����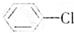 ��
��