��Ŀ����
(16��)��ͼת����ϵ��A��HΪ��ѧ����������ס��ҡ�������Ϊ�������ʣ����мס�������Ϊ���塣��֪�����ҡ�������AΪ��ɫ����ɫ�����塣�Ҿ����������Ӧ������ˮ���ϣ��ǹ�ҵ����H�ķ�Ӧ���̡�B��F�������嶼��ʹ����ʯ��ˮ����ǡ������ַ�Ӧ��������ȥ��
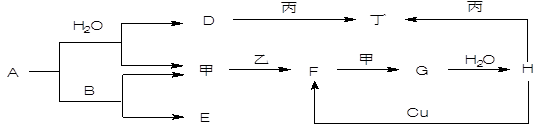
��1��д���������ʵ���ѧʽ��A________ F________ ��_______ ��________
��2��д�����з�Ӧ����ѧ����ʽ��
A��B _________________________________________________________;
��3��д������D��Һ��Ӧ�����ӷ���ʽ ______________________________;
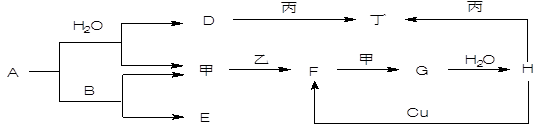
��1��д���������ʵ���ѧʽ��A________ F________ ��_______ ��________
��2��д�����з�Ӧ����ѧ����ʽ��
A��B _________________________________________________________;
��3��д������D��Һ��Ӧ�����ӷ���ʽ ______________________________;
��1����ѧʽNa2O2��SO2��O2��Al
��2��A��B ��2Na2O2 + 2CO2 = 2Na2CO3 + O2��2�֣�
��3��2Al + 2OH- + 2H2O = 2AlO2- + 3H2����2�֣�
��2��A��B ��2Na2O2 + 2CO2 = 2Na2CO3 + O2��2�֣�
��3��2Al + 2OH- + 2H2O = 2AlO2- + 3H2����2�֣�
�������������A+H2O����+D��A+B����+E��������A�ǵ���ɫ��������ж�Ϊ�������ƣ���Ϊ������DΪ�������ƣ��������ƶ�Ϊ������B��F�������嶼��ʹ����ʯ��ˮ����ǣ����ת����ϵ��֪BΪ������̼��FΪ��������EΪ̼���ƣ��Ӽ�+�ҡ�F��F+�ס�G��G+H2O��H��֪��GΪ��������HΪ������ʱ��ȿ��Ժ�D�������Ʒ�Ӧ��Ҳ���Ժ���H��Ӧ˵�����������������ת����ϵ�����������н������жϡ�
����ؼ��Ǵӻ�����A�ǵ���ɫ���塢�������ǻ�ɫ���壬�ƶϳ�AΪNa2O2����ΪS���жϼ�ΪO2��DΪNaOH��FΪ��������SO2��GΪSO3��HΪH2SO4��B��F�������嶼��ʹ����ʯ��ˮ����ǣ������ƶϳ�BΪ������̼CO2��EΪNa2CO3�������ʺ��������Ʒ�Ӧ��Ҳ���Ժ����ᷴӦ��˵������Ϊ�����ۺϷ����жϣ���Ϸ�Ӧ��д��������ӦΪ��
A��Na2O2��+H2O���ף�O2��+D��NaOH����A��Na2O2��+B��CO2�����ף�O2��+E��Na2CO3�����ף�O2��+�ң�S����F��SO2����G��SO3����H��H2SO4����D��NaOH��+����Al��������NaAlO2����H��H2SO4��+����Al��������NaAlO2�����������ĵó������ʷֱ�Ϊ��A��Na2O2��B��CO2��D��NaOH��E��Na2CO3��F��SO2��G��SO3��H��H2SO4���ס�O2���ҡ�S������Al��
��1��A��Na2O2 F��SO2 �ף� O2 ����Al
��2��A��B������Ӧ����̼���ƺ���������ѧ����ʽΪ��2Na2O2+2CO2=2Na2CO3+O2��
��3������D��Һ��Ӧ�ǽ��������������Ʒ�Ӧ����ƫ�����ƺ���������Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
���������⿼����Ԫ�ػ�������ƶϣ��������ʼ��ת����ϵ��������ʵķ��ࡢ��ɫ�����ʺ�ת���������ƶ���ɣ��жϳ������ʶ���ת����ϵ����������𣬹ؼ��������������ʵ�����Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ