��Ŀ����
����Ŀ��ijУ��ѧ�о���ѧϰС���ͬѧ̽����ͭΪ�缫���������Һ�������
��һ��ͬѧ��ͭΪ�缫��ⱥ��ʳ��ˮ��̽���������£�
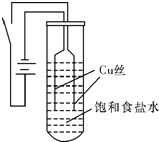
��ʵ�����ͼװ�ã���Դ��ͨ�����ظ���������ͭ˿���д������ݲ�������������������ͭ˿�ɴֱ�ϸ����ʼ30s�ڣ������������ְ�ɫ���ǣ�Ȼ��ʼ���ֳȻ�ɫ���ǣ���ʱ�ⶨ��Һ��pHԼΪ10�����ų������������ӣ��Ȼ�ɫ���������ۼ����Թܵײ�������Һʼ��δ������ɫ��
��ʵ���ʵ������Թܵײ��ijȻ�ɫ����ȡ������װ����֧С�Թ��У������ܿ�ת��Ϊש��ɫ�������IJ������������£�

��ش��������⣺
��1���ۼ����Թܵײ��ijȻ�ɫ�����Ļ�ѧʽΪ______________��
��2�������ĵ缫��ӦʽΪ______________��
��3��д��ʵ����Т١��ڵ����ӷ���ʽ����__________����__________��
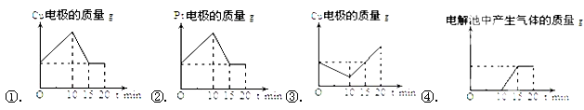
��4���ڶ���ͬѧ��������ȵ�ͭƬ�Ͳ�Ƭ��������ͭ��Һ�У�ͭƬ���Դ����������Ƭ���Դ�����������Ե���ǿ��1Aͨ��10min��Ȼ�ӵ�Դ���Ե���ǿ��2A����ͨ��10min���������б�ʾͭ�缫�����缫�������в�������������͵��ʱ��Ĺ�ϵͼ��������ȷ���ǣ� ��
���𰸡���1��CuOH����2��2Cu+2Cl--2e-=2CuCl����
��3����3Cu2O+2H++2NO3-=6Cu2++2NO��+7H2O����Cu2O+2H+=Cu+Cu2++H2O��
��4���ڢ���
��������
�����������1�����ݳ���ͭ�Ļ�������ɫ�жϣ��Թܵײ��Ȼ�ɫ��������������������ͭ����ѧʽ��CuOH����2�����ʱ��ͭ��������������ͭʧ����������ͭ���ӽ�����Һ����ͭ���Ӻ������������Ȼ���ͭ��ɫ���������Ե缫��ӦʽΪ2Cu+2Cl--2e-=2CuCl����
��3���ٸ���ʵ�����������������ͭ�����ʡ�������ͭ�Ļ�������ɫ�жϣ���������һ������������ͭ��ˮ�����ݵ����غ㡢ԭ���غ㡢����غ㣬�ɵ÷�Ӧ�����ӷ���ʽΪ3Cu2O+2H++2NO3-=6Cu2++2NO��+7H2O���ڸ���ʵ��������ͭ�Ļ�������ɫ����������ͭ������ͭ��ˮ���������ӷ���ʽΪCu2O+2H+=Cu+Cu2++H2O��
��4����������ȵ�ͭƬ�Ͳ�Ƭ������������ͭ��Һ�У�ͭƬ���Դ����������Ƭ���Դ������������CuΪ����������Cu-2e- =Cu2+��PtΪ����������Cu2++2e-=Cu����Cu�缫��������С��Pt�缫���������ӣ�Ȼ�ӵ�Դ��Pt�缫Ϊ��������������Cu�����Է���Cu-2e- =Cu2+��CuΪ����������Cu2++2e-=Cu����Pt�缫�������ּ�С��Cu�缫�����������ù����в��������壬��١��������ҵ���ǿ�ȱ�ԭ���Ĵ��������仯�ij̶ȱ�ԭ���Ĵ�15minʱPt�缫��Cu��ȫ�ܽ��Pt��ʧȥ���ӣ����������ٱ仯����ȻB��Cͼ����ϣ���ڢ���ȷ��



