��Ŀ����
����A��B��C�ڳ����¾�Ϊ��̬���ֱ��ɼס��ҡ������ֶ�����Ԫ����ɣ���Ԫ�ص�ԭ�ӽṹ�д�����������������������1��G�dz����Ľ������ʣ�D�ڳ�����ΪҺ̬��H��һ�ֺ�ɫ���塣�����ʼ��ת����ϵ����ͼ��ʾ(��Ӧ������������ȥ)����ش�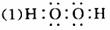
(1)�ס�������Ԫ�ػ����γ�һ�ֺ���4��ԭ�Ӻ˺�18�����ӵĻ�����û�����ĵ���ʽΪ___________��
(2)ʵ������ȡC�����ӷ�Ӧ����ʽΪ_____________��G��D�ڸ����·�Ӧ�Ļ�ѧ����ʽΪ__________________________.
(3)B��C��ȼ�յ�������_____________;��ͨ��״����,lgB��A����ȫȼ��,����Һ̬Dʱ,�ų�142.9kJ������,���ʾBȼ���ȵ��Ȼ�ѧ����ʽΪ_____________;
(4)��ʯīΪ�缫���E��ˮ��Һ����������ӦʽΪ_____________��
(5)ʵ���Ҽ���F��ˮ��Һ���������������ӵIJ�������_____________.
![]()
(2)MnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O 3Fe+4H2O (g)
Mn2++Cl2��+2H2O 3Fe+4H2O (g)![]() Fe3O4+4H2
Fe3O4+4H2
(3)�����������а���ȼ�գ�������ɫ���棻H2(g)+![]() O2 (g)====H2O(1)����H=-285.8kJ��mol
O2 (g)====H2O(1)����H=-285.8kJ��mol
(4)2Cl--2e-====Cl2��
(5)ȡ������Һ����һ֧�Թ��У����뼸��KSCN��Һ���۲���Һ�Ƿ���ɫ�������죬��֤����Fe3+

��ϰ��ϵ�д�
 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ

