��Ŀ����
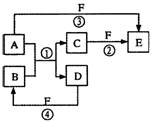
(8��)����A��B��C�ڳ����¾�Ϊ��̬���ֱ��ɼס��ҡ������ֶ�����Ԫ����ɣ���֪��Ԫ�ص�ԭ�ӽṹ�д�����������������������1��������D�ڳ����³�Һ̬��G�dz����Ľ������ʣ�H�dz�������ɫ�����������ʼ��ת����ϵ��ͼ��ʾ(��Ӧ������������ȥ)��

��ش�
(1)��Ӧ�����ӷ���ʽ��_______________________________________________��
(2)�ס�����Ԫ�س��γɻ�����D�⣬�����γ�һ�ֺ���4��ԭ�Ӻ˺�18�����ӵĻ�����û�����ĵ���ʽ��________��
(3)�ڷ�Ӧ���У���֪1 g B��ȫȼ������Һ̬Dʱ���ų�142.9 kJ�����������ʾB��ȼ���ȵ��Ȼ�ѧ����ʽ��_______________________________________________
________________________________________________________________________��
(4)��Ӧ��Ҳ������Ƴ�ԭ���װ�ý��У����ò����缫����KOH��Һ���������Һʱ�������ĵ缫��Ӧʽ��________________________________________________________
________________________________________________________________________��
(5)��Ӧ�ۢ���ͨ������²����Է����У���ͼ�е�����a��________����ʹ��Ӧ�ۢ��ܹ�������װ���У���һ���缫���ϱ�����ͬ��д���õ缫��Ӧʽ________________________________________________________________________��
(1)Cu(OH)2��2H��===Cu2����2H2O
(2)H����������������������������H
(3)H2(g)��O2(g)===H2O(l)
��H����285.8 kJ��mol��1
(4)2H2��4e����4OH��===4H2O
(5)ͨ��(����)��Cu��2e��===Cu2��
���������������Ϣ֪��Ԫ��ΪCl��HΪCu(OH)2��������DΪH2O����AΪO2��BΪH2��CΪCl2��GΪCu��

 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
 ��Ӧ��
��Ӧ��