��Ŀ����
2007��ŵ������ѧ������¹���ѧ��Gerhard Ertl���Ա������ڱ��滯ѧ�����о���ȡ�õĿ����Գɾ͡�ij��Ӧ��Pt/Al2O3/Ba��������������������õĻ�������ͼ��ʾ��ͼ��HC��ʾ̼�⻯���nitrateָ�����Σ����û����о���ָ�� ��
| A������β����ת������ | B������������� |
| C���ϳɰ���ҵ���������� | D������ȼ�յ�ص缫��Ӧ���� |
A
���������������ͼ���֪����Ҫ�ǰ��к�����������һ��������һ����̼ת��Ϊ�����ĵ����Ͷ�����̼����һ����̼��һ������������β������Ҫ�ɷ֣���˴�ѡA
���㣺���黯ѧ������������֪ʶ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д����ڵؿ�����Ҫ��NaIO3����ʽ����,�ں�ˮ����Ҫ��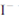 ����ʽ����,��������֮������ͼ��ʾ��ϵ,����ͼʾת����ϵ�Ʋ�����˵������ȷ����
����ʽ����,��������֮������ͼ��ʾ��ϵ,����ͼʾת����ϵ�Ʋ�����˵������ȷ����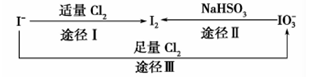
| A������KI������ֽ��ʳ����ӵ������Ƿ��е� |
B������Cl2��ʹʪ���KI������ֽ���ԭ������� |
C����ͼ��֪�����Ե�ǿ��˳��Ϊ |
D��;������������1molI2,��Ӧ��ת�Ƶĵ�����Ϊ10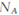 |
������Ŀ�ĵ����������������е�һ�ֶԲ������п�ʴ���Ƴɣ����������ǣ�������
| A������ | B������� | C���ռ� | D������ |
������Һ�У���ʯ��ˮ ��Na2S��Һ ��KMnO4��Һ ����ˮ ���ữ��Ba(NO3)2��Һ
��Ʒ����Һ����������SO2��CO2�������( )
| A��ֻ�Т� | B���٢� | C���٢ڢ� | D���ڢܢ� |
��SO2����ͨ��BaCl2��Һ�У�δ����ɫ����������ͨ����һ����������������������ͨ������岻���ܵ���
| A��Cl2 | B��NH3 | C��H2S | D��CO2 |
ij�����ڷǽ���Ԫ�ص�ԭ�Ӻ��������������Ǵ�����������һ�룬��Ԫ��( )
| A������Ȼ����ֻ�Ի���̬����ʽ���� |
| B�����ʳ������뵼����Ϻ��ά |
| C����������ﲻ���ᷴӦ |
| D����̬�⻯��ȼ����ȶ� |
�ס��ҡ���������Һ������һ��X-(X-ΪCl-��Br-��I-)���ӡ�����мӵ�����Һ����ˮ������Һ��Ϊ��ɫ���ټӱ���Һ����ɫ�����Ա仯����ס��ҡ������κ���( )
| A��Br-��Cl-��I- | B��I-��Br-��Cl- | C��Br-��I-��Cl- | D��Cl-��I-��Br- |
һ������п��100 mL 18.5 mol/L��Ũ�����ַ�Ӧ��п��ȫ�ܽ⣬ͬʱ��������� 33.6 L(��״��)������Ӧ�����Һϡ����1 L�������Һ��c(H+)="0.1" mol/L�������������д������(����)
| A�������ΪSO2��H2�Ļ����� | B���������SO2��H2�����֮��Ϊ4:1 |
| C����Ӧ�й�����97.5 g Zn�������� | D����Ӧ�й�ת��3 mol���� |
 ����ԭ��Mn2+����Ӧ�����Һ�м�������п�ۣ�����ɫ�պ���ʧ��������Һ�ռ�����ƿ�У���ʱ��Һ�Գ����ԡ�
����ԭ��Mn2+����Ӧ�����Һ�м�������п�ۣ�����ɫ�պ���ʧ��������Һ�ռ�����ƿ�У���ʱ��Һ�Գ����ԡ�