��Ŀ����
�¼ұ�������2010����������������������ƹ��Ч���ܼ������ᳫ��̼���������չ����Դ�Ϳ�������Դ�������й�������˲�������ǣ� ��
| A�����������ḻ��ú̿��Դ�����ٶ�ʯ�͵����� |
| B�����������غ������˽���������վ�������Դ���� |
| C��������ܶೡ�ݵ����ʹ�÷Ǿ��象Ĥ���Գ������̫���ܣ����ֵ�̼���� |
| D������ͣ������װ�������ʩ���ɽ�����β����CO��NOx��Ӧ���������� |
A
�������������A.����ú̿��Դ����Ȼ���Լ��ٶ�ʯ�͵���������ú̿Ҳ�ǻ�ʯ��Դ����ʹ�ù����л���������Ķ�����̼���嵼������ЧӦ�������ķ۳��ᵼ�����������ķ���������B�����������غ�����������Դ�ḻ���˽���������վ���ͽ�ȡ�ģ���ɽ��ɽ�����Ժ��������Դ���⡣��ȷ��C��������ܶೡ�ݵ����ʹ�÷Ǿ��象Ĥ���������̫���ܣ���ʡ������Դ�Ŀ������ã����ֵ�̼���õ�˼�롣��ȷ��D������������β���к���CO��NOx��������ͣ������װ�������ʩ���ɽ�CO��NOx����Ӧת��Ϊ����������CO2��N2.��ȷ��
���㣺���黯ѧ�ڽ��ܼ��š����������е���Ҫ���á�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д��״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ����
�� CH3OH(g)��H2O(g)=CO2(g)��3H2(g) ��H����49.0 kJ/mol
�� CH3OH(g)��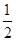 O2(g)=CO2(g)��2H2(g) ��H����192.9 kJ/mol
O2(g)=CO2(g)��2H2(g) ��H����192.9 kJ/mol
������˵����ȷ���ǣ�������
| A��CH3OH��ȼ����Ϊ192.9 kJ/mol |
B����Ӧ���е������仯��ͼ��ʾ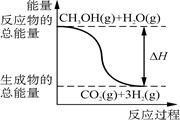 |
| C��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
D�����ݢ���֪��Ӧ��CH3OH(l)��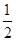 O2(g)=CO2(g)��2H2(g)�Ħ�H>��192.9 kJ/mol O2(g)=CO2(g)��2H2(g)�Ħ�H>��192.9 kJ/mol |
����˵���������
| A���ɵ���Aת��Ϊ����B����H��+119kJ/mol,��֪����A�ȵ���B�ȶ� |
| B�����ȵ�̼��CO2��Ӧ����������ԭ��Ӧ���������������������ڷ�Ӧ���������ķ�Ӧ |
| C��������ѧ��Ӧ���ʵĸ��������Dzμӷ�Ӧ�ĸ����ʵĽṹ������ |
| D������һ�����淴Ӧ����H��0���ﵽƽ��������¶ȿ������ӻ���ӵİٷ����������Ч��ײ�Ĵ�����ʹV������ı�������V������ı���,�Ӷ�ʹ��ѧ��Ӧƽ�������ƶ� |
����˵����ȷ����
| A��������һ�ָ�Ч��û����Ⱦ��һ����Դ |
| B��̫���ܡ����ܡ����ܡ������ܡ������ܺ��������ܵȶ�������Դ |
| C��CaCO3��s����CaO��s����CO2��g�������²����Է����У�˵���÷�Ӧ�Ħ�H��0 |
| D��ˮ�����ӻ�����Kw�����¶ȵ����߶�����˵��ˮ�ĵ����Ƿ��ȷ�Ӧ |
����ᣨHF����һ�����ᡣ25��ʱ����20mL0.1mol/L������м���VmL 0.1mol/LNaOH��Һ��ַ�Ӧ����֪��
HF��aq����OH����aq����F����aq����H2O��l�� ��H����67.7kJ/mol
H����aq����OH����aq����H2O��l�� ��H����57.3kJ/mol
�������⣬�����жϻ������ȷ����
| A�������ĵ�����������ȵ� |
| B����V��20ʱ����Һ�У�c��F������c��Na������0.1mol/L |
| C����V��20ʱ����Һ������Ũ�ȹ�ϵ����Ϊ��c��Na������c��F���� |
| D����V��20ʱ����Һ������Ũ�ȹ�ϵһ��Ϊ��c��Na������c��F������c��OH������c��H���� |
ǿ����ǿ���ϡ��Һ�����кͷ�Ӧ����ЧӦ��
H+(aq)+ OH��(aq) �� H2O (l) +57.3kJ
��1L0.5mol/L��NaOH��Һ�м���ϡ���ᡢŨ���ᡢϡ���ᣬ��ǡ����ȫ��Ӧʱ����ЧӦQ1��Q2��Q3�Ĺ�ϵ��ȷ����
| A��Q1 < Q2 < Q3 | B��Q1 > Q3 > Q2 | C��Q2 > Q1 > Q3 | D��Q1 < Q3 < Q2 |
��ͬ��ͬѹ��,���и����Ȼ�ѧ����ʽ�У���H2>��H1����
| A��S(g)+O2(g)��SO2(g)����H1�� S(s)+O2(g)��SO2(g)����H2 |
| B��2H2(g)+O2(g)��2H2O(g)����H1�� 2H2(g)+O2(g)��2H2O(l)����H2 |
C��C(s)+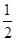 O2(g)=CO(g)��H1��C(s)+O2(g)=CO2(g) ��H2 O2(g)=CO(g)��H1��C(s)+O2(g)=CO2(g) ��H2 |
| D��H2(g)+Cl2(g)��2HCl(g)����H1�� 2H2(g)+2Cl2(g)��4HCl(g)����H2 |
��298 K��100 kPaʱ����֪��
�� 2H2O(g)=O2(g)��2H2(g)����H1 �� Cl2(g)��H2(g)=2HCl(g) ��H2
�� 2Cl2(g)��2H2O(g)=4HCl(g)��O2(g) ��H3
��H3�릤H1�ͦ�H2��Ĺ�ϵ��ȷ����(����)
| A����H3����H1��2��H2 | B����H3����H1����H2 | C����H3����H1��2��H2 | D����H3����H1����H2 |
��֪��Ӧ��H2(g) �� 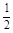 O2(g) �� H2O(g) ��H1
O2(g) �� H2O(g) ��H1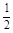 N2(g) �� O2(g) �� NO2 (g) ��H2
N2(g) �� O2(g) �� NO2 (g) ��H2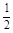 N2(g) ��
N2(g) �� 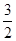 H2(g) �� NH3 (g) ��H3
H2(g) �� NH3 (g) ��H3
��Ӧ2NH3 (g) ��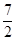 O2(g) ��2NO2 (g) ��3H2O(g) �ġ�H��
O2(g) ��2NO2 (g) ��3H2O(g) �ġ�H��
| A��2��H1��2��H2��2��H3 | B����H1����H2����H3 |
| C��3��H1��2��H2��2��H3 | D��3��H1��2��H2��2��H3 |