��Ŀ����
��֪��Ӧ��H2(g) �� 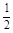 O2(g) �� H2O(g) ��H1
O2(g) �� H2O(g) ��H1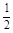 N2(g) �� O2(g) �� NO2 (g) ��H2
N2(g) �� O2(g) �� NO2 (g) ��H2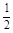 N2(g) ��
N2(g) �� 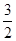 H2(g) �� NH3 (g) ��H3
H2(g) �� NH3 (g) ��H3
��Ӧ2NH3 (g) ��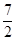 O2(g) ��2NO2 (g) ��3H2O(g) �ġ�H��
O2(g) ��2NO2 (g) ��3H2O(g) �ġ�H��
| A��2��H1��2��H2��2��H3 | B����H1����H2����H3 |
| C��3��H1��2��H2��2��H3 | D��3��H1��2��H2��2��H3 |
D
�����������������ʽ�١�3+�ڡ�2���ۡ�2�õ���Ӧ2NH3 (g) ��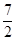 O2(g) ��2NO2 (g) ��3H2O(g) �����Ը÷�Ӧ�ġ�H��3��H1��2��H2��2��H3��
O2(g) ��2NO2 (g) ��3H2O(g) �����Ը÷�Ӧ�ġ�H��3��H1��2��H2��2��H3��
���㣺�����˹���ɵ�Ӧ���й����⡣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д��¼ұ�������2010����������������������ƹ��Ч���ܼ������ᳫ��̼���������չ����Դ�Ϳ�������Դ�������й�������˲�������ǣ� ��
| A�����������ḻ��ú̿��Դ�����ٶ�ʯ�͵����� |
| B�����������غ������˽���������վ�������Դ���� |
| C��������ܶೡ�ݵ����ʹ�÷Ǿ��象Ĥ���Գ������̫���ܣ����ֵ�̼���� |
| D������ͣ������װ�������ʩ���ɽ�����β����CO��NOx��Ӧ���������� |
��ͼ��ʾ��298 KʱN2��H2��Ӧ�����������仯������ͼ������������ȷ����(�� ��) 
A���÷�Ӧ���Ȼ�ѧ����ʽΪ��N2��3H2 2NH3����H����92 kJ/mol 2NH3����H����92 kJ/mol |
| B��a�����Ǽ������ʱ�������仯���� |
| C������������û�ѧ��Ӧ�ķ�Ӧ�ȸı� |
| D�����¶ȡ����һ���������£�ͨ��1 mol N2��3 mol H2��Ӧ��ų�������ΪQ1 kJ����ͨ��2 mol N2��6 mol H2��Ӧ��ų�������ΪQ2 kJ����184��Q2��2Q1 |
��֪����H��(aq)��OH��(aq)=H2O(l)����H1����2SO2(g)��O2(g) 2SO3(g)����H2��������������ʱ�����ӷ�Ӧ��������������ж���ȷ����(�� ��)
2SO3(g)����H2��������������ʱ�����ӷ�Ӧ��������������ж���ȷ����(�� ��)
| A����H1����H2��С | B����H1����H2���� |
| C����H1��С����H2��С | D����H1���䣬��H2���� |
25�桢101kPa�£�2g����ȼ������Һ̬ˮ���ų�285��8 kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽ��ȷ����
| A��2H2��g����O2��g����2H2O��l����H����285��8 kJ��mol |
| B��2H2��g����O2��g����2H2O��l����H����571��6 kJ��mol |
| C��2H2��g����O2��g����2H2O��g����H����571��6kJ��mol |
D��H2��g����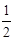 O2��g����H2O��l����H����285��8 kJ��mol O2��g����H2O��l����H����285��8 kJ��mol |
��֪�����Ȼ�ѧ����ʽ��
Zn��s�� + 1/2 O2��g��= ZnO��s�� ��H= -351��1 kJ��mol-1 ��
Hg��l�� + 1/2 O2��g��= HgO��s�� ��H= -90��7 kJ��mol-1 ��
�ɴ˿�֪Zn��s��+ HgO��s��= ZnO��s��+ Hg��l���ķ�Ӧ�Ȧ�HΪ
| A��-260��4 kJ��mol-1 | B��+260��4 kJ��mol-1 | C��- 441��8 kJ��mol-1 | D��+ 441��8 kJ��mol-1 |
�����뻯ѧ��Ӧ�����仯��ص���������ȷ����
| A����֪CH4(g)��2O2(g)=CO2(g)��2H2O(g) ��H��-802 kJ/mol�������ȼ����Ϊ802 kJ/mol |
| B������H2��O2����ȫȼ�գ�����H2O(g)������H2O(l)�ų��������� |
| C��ͬ��ͬѹ�£�H2(g)��Cl2(g)=2HCl(g)�ڹ��պ͵�ȼ�����µĦ�H��ͬ |
| D����ʯī�Ƚ��ʯ�ȶ���֪��C(���ʯ, s)=C(ʯī, s) ��H<0 |
CH4��H2��CO��ȼ���ȷֱ�Ϊ890.31kJ/mol��285.8kJ/mol��110.5 kJ/mol�������Ȼ�ѧ����ʽ��д��ȷ����
| A��CH4(g)+2O2(g)=CO2(g)+2H2O(g)��H=-890.31kJ/mol |
| B��2H2(g)+ O2(g)= 2H2O(l)��H=-285.8kJ/mol |
| C��CO (g)+ H2O(g)= CO2(g)+ H2 (g)��H=+175.3kJ/mol |
| D��2CO (g)+ O2(g) = 2CO2(g)��H="-221" kJ/mol |
�����Ȼ�ѧ����ʽ��ȷ���ǣ�ע��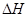 �ľ���ֵ����ȷ��( )
�ľ���ֵ����ȷ��( )
| A��C2H5OH(l)+3O2(g)=2CO2(g) +3H2O(g)����H=" ��1367.0" kJ/mol��ȼ���ȣ� |
| B��NaOH(aq) + HCl(aq)="NaCl(aq)" + H2O(l)����H= ��57.3kJ/mol���к��ȣ� |
| C��S(s) + O2(g) = SO2(g)����H= ��269.8kJ/mol����Ӧ�ȣ� |
| D��2HCl(g)=Cl2(g) + H2(g)����H=" ��" 184.6kJ/mol����Ӧ�ȣ� |