��Ŀ����
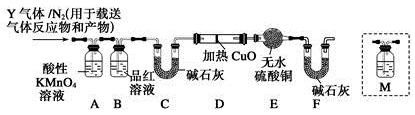
��10�֣���ͼ���о�ͭ��Ũ����ķ�Ӧװ�ã� 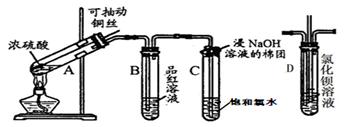
��1��A�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ӧһ��ʱ��ɹ۲쵽B�Թ��е�����Ϊ ��
��3��C�Թܿڽ���NaOH��Һ������������ ��
��4��ʵ�������֤��A�Թ��з�Ӧ���ò����Ƿ���ͭ���ӵIJ��������� ��
��5����ͭ��Ũ���ᷴӦ�Ĺ����У������к�ɫ���ʳ��֣�������������������ϡ�
| ����1 | 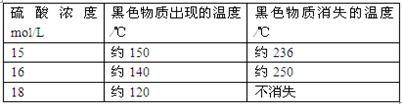 ����ͭ��Ũ���ᷴӦ������ɫ���ʵ�������� |
| ����2 | X���߾������������ͭ��Ũ���ᷴӦ���ɵĺ�ɫ����ΪCu2S��CuS��Cu7S4�е�һ�ֻ��֡� |
A��ͭ��Ũ���ᷴӦʱ���漰�ķ�Ӧ���ܲ�ֹһ�� B������Ũ��ѡ���ʵ����ɱ����������г��ֺ�ɫ���ʣ�C���÷�Ӧ����������֮һ������Ũ�ȡ�15 mol��L D������Ũ��Խ��ɫ����Խ����֡�Խ����ʧ
��1��Cu+2H2SO4(Ũ) CuSO4+SO2��+2H2O ����2����Һ�ɺ�ɫ�����ɫ
CuSO4+SO2��+2H2O ����2����Һ�ɺ�ɫ�����ɫ
��3������Cl2��SO2����ֹ��Ⱦ��������4��������ͭ˿����ֹ��Ӧ����ȴ��A����Һ��������ʢ������ˮ���ձ����Թܣ���۲���Һ��ɫ�Ƿ�Ϊ��ɫ����5��A��B��D
���������������1����A�Թ���Cu���ȵ�Ũ���ᷢ����Ӧ��Cu+2H2SO4(Ũ) CuSO4+SO2��+2H2O ����2�����ڷ�Ӧ������SO2���壻��������Ư���ԣ���ʹƷ����Һ��ɫ����˷�Ӧһ��ʱ��ɹ۲쵽B�Թ���Ʒ����Һ�ɺ�ɫ��Ϊ��ɫ����3��Cl2��SO2���������´�����Ⱦ����ΪSO2��Cl2�������������壬������C�Թܿ��ý���NaOH��Һ��������������ɢ��SO2�� Cl2���塣��C�Թ��з�����Ӧ��Cl2��SO2��2H2O=H2SO4��2HCl����4��ʵ���������Ҫ֤��A�Թ��з�Ӧ���ò��ﺬ��ͭ���ӣ�����������������ͭ˿����ֹ��Ӧ����ȴ��A����Һ��������ʢ������ˮ���ձ����Թܣ���۲���Һ��ɫ�Ƿ�Ϊ��ɫ����5�����������ṩ����Ϣ��֪��ͭ��Ũ���ᷴӦʱ���漰�ķ�Ӧ���ܲ�ֹһ����������Ũ��ѡ���ʵ����ɱ����������г��ֺ�ɫ���ʣ�����Ũ��Խ��ɫ����Խ����֡�Խ����ʧ�����������Ũ���Ƕ��ٲ�����ȷ���������ȷѡ����A��B��D��
CuSO4+SO2��+2H2O ����2�����ڷ�Ӧ������SO2���壻��������Ư���ԣ���ʹƷ����Һ��ɫ����˷�Ӧһ��ʱ��ɹ۲쵽B�Թ���Ʒ����Һ�ɺ�ɫ��Ϊ��ɫ����3��Cl2��SO2���������´�����Ⱦ����ΪSO2��Cl2�������������壬������C�Թܿ��ý���NaOH��Һ��������������ɢ��SO2�� Cl2���塣��C�Թ��з�����Ӧ��Cl2��SO2��2H2O=H2SO4��2HCl����4��ʵ���������Ҫ֤��A�Թ��з�Ӧ���ò��ﺬ��ͭ���ӣ�����������������ͭ˿����ֹ��Ӧ����ȴ��A����Һ��������ʢ������ˮ���ձ����Թܣ���۲���Һ��ɫ�Ƿ�Ϊ��ɫ����5�����������ṩ����Ϣ��֪��ͭ��Ũ���ᷴӦʱ���漰�ķ�Ӧ���ܲ�ֹһ����������Ũ��ѡ���ʵ����ɱ����������г��ֺ�ɫ���ʣ�����Ũ��Խ��ɫ����Խ����֡�Խ����ʧ�����������Ũ���Ƕ��ٲ�����ȷ���������ȷѡ����A��B��D��
���㣺����Cu��Ũ���ᷢ����ԭ��������������������ӵļ��顢��ѧ����ʽ����д��֪ʶ��

������H2S����ĵ��ܵ�ȼ������ʢ��һ����O2�ļ���ƿ�ڣ�����3����Ӧ�������Ⱥ�˳����
�� 2H2S + O2 �� 2S + 2H2O �� 2H2S + SO2 �� 3S + 2H2O �� 2H2S + 3O2 �� 2SO2 + 2H2O
| A���٢ڢ� | B���ۢ٢� | C���٢ۢ� | D���ۢڢ� |
��������������Ⱦ��Ϊ���أ����������������ü�����⡣
��1���������糧Ϊ��ȥ�к�����SO2�������Ϊ����������β�������գ���ͼ��ʾ��д��β���������з�Ӧ�Ļ�ѧ����ʽ�� ��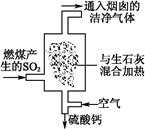
��2�������Ļ������糧ͨ�����ں��ߣ�һ�㺣ˮ�������ԣ���Ҫ����Na+��Mg2+��K+��Ca2+��Cl-��Br-��SO42-��HCO3-�����ӡ�����SO2������Ҳ�������ú�ˮ�����乤����������ͼ��ʾ��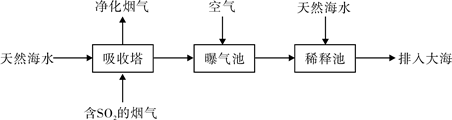
����������ͨ�������Ŀ���� ��
��ͨ���������������еĺ�ˮ����Ȼ��ˮ��ȣ�Ũ�������Բ�ͬ�������� ��
| A��Cl- | B��Na+ | C��Mg2+ | D��HCO3- |
�輰�仯����������ִ������ķ�չ��������ס���ش������й����⣺
(1)��ԭ�ӵĽṹʾ��ͼ��________��
(2)������Ʒ���豸���õIJ������ڹ����ε���________��
�ٳ�����Ͽˮ���ӡ���ʯӢ���ά�����մ�����
����ͨ�������ݹ�̫���ܵ��
| A���٢ڢ� | B���ۢܢ� | C���ڢۢ� | D���٢ۢ� |

 H��+Cl��+ HClO����ƽ�ⳣ������ʽΪK�� ��
H��+Cl��+ HClO����ƽ�ⳣ������ʽΪK�� ��
