��Ŀ����
18��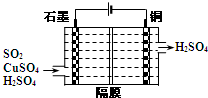 ��������������������Ư����Ҳ��һ����Ҫ�Ĺ�ҵԭ�ϣ�
��������������������Ư����Ҳ��һ����Ҫ�Ĺ�ҵԭ�ϣ���1����a mol SO2ͨ��1L 1mol/L NaOH��Һ�г�ַ�Ӧ������������D����������������ѡ��ǰ����ĸ��
A��a��1ʱ���� B��a=1ʱ���� C������ D��С��
��2�����շۣ�Na2S2O4���㷺����ӡȾ��ҵ��ʳƷ������ҵ�����Խ�SO2ͨ������ƣ�Na2C2O4����NaOH�Ļ����Һ����ȡ���շۣ���ȡ���շ۵����ӷ���ʽΪ2SO2+C2O42-+4OH-$\frac{\underline{\;\;��\;\;}}{\;}$S2O42-+2CO32-+2H2O��
��3����ҵ�ϳ���SO2Ϊԭ����ȡNa2S2O3���������£�
�ٽ�Na2S��Na2CO3��2��1�����ʵ���֮����ɻ����Һ��
�ڽ������Һע�볨�ڷ�Ӧ���У����ȷ�Ӧ�����¶ȿ�����50�����ң�
����Ӧ���л���ͨ��SO2��������ʹNa2S��Na2CO3��ȫ��Ӧ��
�ܷ�Ӧ���������Ũ����Һ����ȴ��30��������������Na2S2O3���壮
���˳����壬ĸҺѭ�����ã�
�ݴ���ش�
��������ҵ��ȡNa2S2O3�ķ�Ӧ�Ļ�ѧ����ʽΪ4SO2+2Na2S+Na2CO3$\frac{\underline{\;ͨ��\;}}{\;}$3Na2S2O3+CO2
��������ҵ��������ҪĿ����ӯ����Ϊ��Լ�ɱ������ٶԻ�������Ⱦ��Ӧ�����ܵ���߲��ʣ���ȡ��������������һ˼����Т٢ۢݣ������ţ�
��������ҵ������Na2S2O3�����������ʣ�������������Na2SO3��Na2SO4�����������п��������Ʒ��Na2S2O3�����ʵ�����������A������ѡ��ǰ����ĸ��
A����Ʒ����ԭ�Ӻ���ԭ�ӵ����ʵ���֮��
B����Ʒ����ԭ�Ӻ���ԭ�ӵ����ʵ���֮��
C����Ʒ����ԭ�Ӻ���ԭ�ӵ����ʵ���֮��
D�����Ͼ���
��4��һ����ͭ�����������������������̣�
����ͭ����������ɹ�ҵβ����SO2�IJ��ִ���������������ӦΪ��2SO2+2n Cu+��n+1��O2+��2-2n�� H2O=2n CuSO4+��2-2n�� H2SO4
ÿ���ձ�״����11.2L SO2����SO2��ԭ��O2������Ϊ8g��
��������ͼ��ʾ�绯ѧװ��������һ����SO2�������Cu��������д��װ������������Ӧ�����ӷ���ʽCu2++SO2+2H2O$\frac{\underline{\;���\;}}{\;}$Cu+SO42-+4H+��
���� ��1��SO2ͨ��NaOH��Һ�����ݵ���غ���c��Na+��+c��H+��=c��HSO3-��+2c��SO32-��+c��OH-�����ݴ˴��⣻
��2����SO2ͨ������ƣ�Na2C2O4����NaOH�Ļ����Һ����Na2S2O4������Ԫ���غ��֪���ﻹ��̼���ƺ�ˮ�����ݵ���غ���д���ӷ���ʽ��
��3������������������Ϣ����ҵ����Na2S��Na2CO3�Լ�SO2��ȡNa2S2O3������Ԫ���غ��д����Ӧ�Ļ�ѧ����ʽ��
������Ϊ��Լ�ɱ������ٶԻ�������Ⱦ�����Խ�������������ĸҺѭ��ʹ�ã����ݷ�Ӧ����ʽ��Ͷ�������֮�ȵȷ�Ӧ�еļ�����֮�ȣ���SO2����������߲��ʣ��ݴ˴��⣻
������Na2CO3��Һ���������Ӧ�������������ƣ��������ƿɱ������������ƣ��Ƚ�Na2S2O3��Na2SO3��Na2SO4�Ļ�ѧʽ��֪��Na2S2O3��Na2SO3��Na2SO4������������ԭ������ԭ�����ʵ���֮�Ȳ�ͬ����Na2SO3��Na2SO4����ԭ������ԭ�����ʵ���֮����ͬ����Na2S2O3��Na2SO3����ԭ������ԭ�����ʵ���֮����ͬ��Na2S2O3����ԭ������ԭ�����ʵ���֮�Ƚ���Na2SO3��Na2SO4֮�䣬�ݴ�ѡ��
��4���ٸ��ݵ��ӵ�ʧ�غ���㱻SO2��ԭ��O2��������
��ͼ�е�װ��Ϊ���أ�������ͭ���ӵõ�������ͭ�������϶�������ʧ����������������ӣ�
��� �⣺��1��SO2ͨ��NaOH��Һ�����ݵ���غ���c��Na+��+c��H+��=c��HSO3-��+2c��SO32-��+c��OH-����������c��Na+��+c��H+����c��HSO3-��+c��SO32-��+c��OH-������ѡD��
��2����SO2ͨ������ƣ�Na2C2O4����NaOH�Ļ����Һ����Na2S2O4������Ԫ���غ��֪���ﻹ��̼���ƺ�ˮ����Ӧ�����ӷ���ʽΪ2SO2+C2O42-+4OH- $\frac{\underline{\;\;��\;\;}}{\;}$S2O42-+2CO32-+2H2O��
�ʴ�Ϊ��2SO2+C2O42-+4OH- $\frac{\underline{\;\;��\;\;}}{\;}$S2O42-+2CO32-+2H2O��
��3������������������Ϣ����ҵ����Na2S��Na2CO3�Լ�SO2��ȡNa2S2O3����Ӧ�Ļ�ѧ����ʽΪ4SO2+2Na2S+Na2CO3 $\frac{\underline{\;ͨ��\;}}{\;}$3Na2S2O3+CO2��
�ʴ�Ϊ��4SO2+2Na2S+Na2CO3 $\frac{\underline{\;ͨ��\;}}{\;}$3Na2S2O3+CO2��
������Ϊ��Լ�ɱ������ٶԻ�������Ⱦ�����Խ�������������ĸҺѭ��ʹ�ã����ݷ�Ӧ����ʽ��Ͷ�������֮�ȵȷ�Ӧ�еļ�����֮�ȣ���SO2����������߲��ʣ�������ȡ���������֡�ӯ������Լ�ɱ������ٶԻ�������Ⱦ�������ܵ���߲��ʡ���һ˼����Т٢ۢݣ�
�ʴ�Ϊ���٢ۢݣ�
������Na2CO3��Һ���������Ӧ�������������ƣ��������ƿɱ������������ƣ�����������Na2SO3��Na2SO4���Ƚ�Na2S2O3��Na2SO3��Na2SO4�Ļ�ѧʽ��֪��Na2S2O3��Na2SO3��Na2SO4������������ԭ������ԭ�����ʵ���֮�Ȳ�ͬ����Na2SO3��Na2SO4����ԭ������ԭ�����ʵ���֮����ͬ����Na2S2O3��Na2SO3����ԭ������ԭ�����ʵ���֮����ͬ��Na2S2O3����ԭ������ԭ�����ʵ���֮�Ƚ���Na2SO3��Na2SO4֮�䣬���Ը��ݲ�Ʒ����ԭ�Ӻ���ԭ�ӵ����ʵ���֮�ȿ�ȷ��Na2S2O3�����ʵ�����������ѡA��
�ʴ�Ϊ��Na2SO4��A��
��4�����ڷ�Ӧ��2SO2+2n Cu+��n+1��O2+��2-2n�� H2O=2n CuSO4+��2-2n�� H2SO4 �У�ÿĦ����������ʧȥ2mol���ӣ���ÿĦ�������ܵõ�4mol���ӣ�����1molSO2�ܻ�ԭ0.5molO2��ÿ���ձ�״����11.2L��0.5mol SO2����SO2��ԭ��O2������Ϊ0.25mol��32g/mol=8g��
�ʴ�Ϊ��8��
��ͼ�е�װ��Ϊ���أ�������ͭ���ӵõ�������ͭ�������϶�������ʧ����������������ӣ���Ӧ����ʽΪCu2++SO2+2H2O$\frac{\underline{\;���\;}}{\;}$ Cu+SO42-+4H+��
�ʴ�Ϊ��Cu2++SO2+2H2O$\frac{\underline{\;���\;}}{\;}$ Cu+SO42-+4H+��
���� ������Ҫ����Ԫ�ػ�����֪ʶ�빤ҵӦ�ã��е��Ѷȣ��漰֪ʶ��϶࣬�ۺ���ǿ������ʱע��Ԫ�ػ��������֪ʶ�ͻ�ѧԭ��֪ʶ��������ã�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| A�� | ������K�պϺ�����ָ�뷢��ƫת��֤��HCl�ǵ���� | |
| B�� | �պϿ���K�����ձ��м���NaCl���壬����HCl��NaCl����Ӧ���ʵ�����ָ�벻�����仯 | |
| C�� | �պϿ���K������Һ�м���CaCO3���壬������ʾ���������� | |
| D�� | �պϿ���K������Һ�м���NaOH���壬������ʾ���������� |
| A�� | HClO+SO2+H2O�THCl+H2SO4������ HClO��H2SO4 | |
| B�� | Al2O3+2NaOH�T2NaAlO2+H2O��Al2O3������������ | |
| C�� | NH3+H3O+�TNH4++H2O��NH3���H+��������H2Oǿ | |
| D�� | ��֪C��s��ʯī���TC��s�����ʯ����H=+1.9 kJ/mol�����ʯ��ʯī�ȶ� |
| A�� | ú�ĸ����ǻ�ѧ�仯 | |
| B�� | ʯ�ͺ���Ȼ������Ҫ�ɷֶ���̼�⻯���� | |
| C�� | ʯ���ѻ��õ��������Ǵ����� | |
| D�� | ú���͵ķ�����Ի�ø��ַ����� |
| A�� | �ܢ٢ۢ� | B�� | �ܢ٢ۢ� | C�� | �ܢۢܢ� | D�� | �٢ڢܢ� |
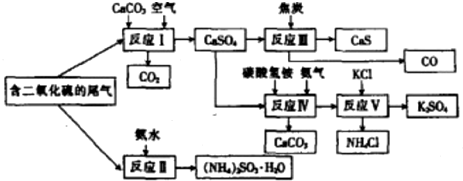
 ʵ�����а���һ��˳����2mLŨ���ᡢ3mL�Ҵ���2mL���������Ʊ�����������������������������ͨ������̼������Һ��Һ���ϣ���ͼ��ʾ����
ʵ�����а���һ��˳����2mLŨ���ᡢ3mL�Ҵ���2mL���������Ʊ�����������������������������ͨ������̼������Һ��Һ���ϣ���ͼ��ʾ����
 ����
����
 ��
��