��Ŀ����
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ�������ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
��1����֪��N2(g)+O2(g)=2NO(g)����H= +180.5kJ/mol
 N2(g)+3H2(g)
N2(g)+3H2(g) 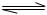 2NH3(g)����H= ��92.4kJ/mol
2NH3(g)����H= ��92.4kJ/mol
2H2(g)+O2(g)=2H2O(g) ����H= ��483.6kJ/mol
����������������һ�����������ˮ������Ӧ���Ȼ�ѧ����ʽΪ ��
��2�����ݻ��̶����ܱ������з������������Ĵ�������Ӧ�������ڲ������ʵ����ʵ���Ũ�����±���
����ʼʱ���ϱ�c (O2)��c (NH3)��1.25����ԭ���� ��
�ڷ�Ӧ�ڵ�2min����4minʱO2��ƽ����Ӧ����Ϊ ��
�۷�Ӧ�ڵ�2min�ı��˷�Ӧ�������ı������������ ��
������Ӧ�ڵ�6min����ı�һ�ַ�Ӧ���������ı���������жϵ�������
��
��1����֪��N2(g)+O2(g)=2NO(g)����H= +180.5kJ/mol
 N2(g)+3H2(g)
N2(g)+3H2(g)  2NH3(g)����H= ��92.4kJ/mol
2NH3(g)����H= ��92.4kJ/mol2H2(g)+O2(g)=2H2O(g) ����H= ��483.6kJ/mol
����������������һ�����������ˮ������Ӧ���Ȼ�ѧ����ʽΪ ��
��2�����ݻ��̶����ܱ������з������������Ĵ�������Ӧ�������ڲ������ʵ����ʵ���Ũ�����±���
| ʱ�䣯Ũ�� | c(NH3)(mol/L) | C(O2)(mol/L) | C(NO)(mol/L) |
| ��ʼ | 0.8 | 1.6 | 0 |
| ��2min | 0.6 | a | 0.2 |
| ��4min | 0.3 | 0.975 | 0.5 |
| ��6min | 0.3 | 0.975 | 0.5 |
| ��8min | 0.54 | 0.9 | 0.56 |
�ڷ�Ӧ�ڵ�2min����4minʱO2��ƽ����Ӧ����Ϊ ��
�۷�Ӧ�ڵ�2min�ı��˷�Ӧ�������ı������������ ��
������Ӧ�ڵ�6min����ı�һ�ַ�Ӧ���������ı���������жϵ�������
��
��1��4NH3(g)+5O2(g)  4NO(g)+6H2O (g)����H=-905kJ��mol��1 ��3�֣�
4NO(g)+6H2O (g)����H=-905kJ��mol��1 ��3�֣�
��2���ٿ�����NH3��ת���ʣ�2�֣� ��0.1875 mol/��L��min�� ��2�֣�
��ʹ�ô�����2�֣� �����¶ȣ�2�֣�
������NH3��Ũ�ȣ���2�֣�
��Ϊ6min��ƽ��������Ӧ�����ƶ�����NH3��Ũ���������ˡ���1�֣�
 4NO(g)+6H2O (g)����H=-905kJ��mol��1 ��3�֣�
4NO(g)+6H2O (g)����H=-905kJ��mol��1 ��3�֣���2���ٿ�����NH3��ת���ʣ�2�֣� ��0.1875 mol/��L��min�� ��2�֣�
��ʹ�ô�����2�֣� �����¶ȣ�2�֣�
������NH3��Ũ�ȣ���2�֣�
��Ϊ6min��ƽ��������Ӧ�����ƶ�����NH3��Ũ���������ˡ���1�֣�
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
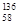 Ce��
Ce��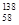 Ce��
Ce��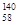 Ce��
Ce��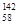 Ce�����ǻ�Ϊͬ��������
Ce�����ǻ�Ϊͬ��������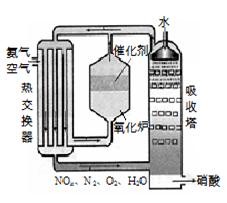
 4NO(g) +6H2O(l) ��H��0
4NO(g) +6H2O(l) ��H��0 m3�������蹤ҵ������������У�ͨ��ѭ����������ʹNH3��O2������ȫ���á�
m3�������蹤ҵ������������У�ͨ��ѭ����������ʹNH3��O2������ȫ���á�
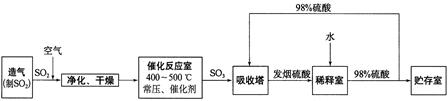
 2SO3(g)���о����֣�SO3���������(SO3%)���¶�(T)�ı仯�����ߢ���ʾ�������ж���ȷ����________
2SO3(g)���о����֣�SO3���������(SO3%)���¶�(T)�ı仯�����ߢ���ʾ�������ж���ȷ����________
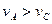
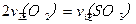
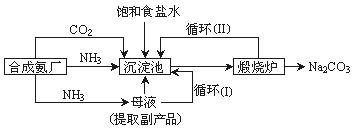
 Na2CO3���ڹ�ҵ����������������ҪӦ�ã���ʵ�����Ʒ���ҵ�Ʒ����£�
Na2CO3���ڹ�ҵ����������������ҪӦ�ã���ʵ�����Ʒ���ҵ�Ʒ����£� Na2CO3����
Na2CO3����