��Ŀ����
����ѧ����ѧ�뼼������12�֣����Ṥҵ����������ʾ��
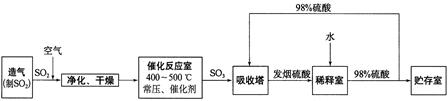
��1������Ӧ�ҷ�����Ӧ�Ļ�ѧ����ʽ�ǣ� ���÷�Ӧͨ����V2O5 ��������������������ǣ�V2O5 ����SO2 ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2 ������д���ô�ѭ�������Ļ�ѧ����ʽ��
��
��2����������ͼ�ж�����˵����ȷ���� ������ĸ����
a���������������SO2 ��ת����
b��ʹ�ô��������SO2 �ķ�Ӧ���ʺ�ת����
c����98%����������SO3 �����Ա����γ����������������
��3��ÿ160g SO3 ������H2O(l)���Ϸų�260.6kJ���������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��4���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�������������˷�ֹSO2 ��Ⱦ�������õ�����⣬��ҪĿ���ǣ� ��

��1������Ӧ�ҷ�����Ӧ�Ļ�ѧ����ʽ�ǣ� ���÷�Ӧͨ����V2O5 ��������������������ǣ�V2O5 ����SO2 ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2 ������д���ô�ѭ�������Ļ�ѧ����ʽ��
��
��2����������ͼ�ж�����˵����ȷ���� ������ĸ����
a���������������SO2 ��ת����
b��ʹ�ô��������SO2 �ķ�Ӧ���ʺ�ת����
c����98%����������SO3 �����Ա����γ����������������
��3��ÿ160g SO3 ������H2O(l)���Ϸų�260.6kJ���������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��4���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�������������˷�ֹSO2 ��Ⱦ�������õ�����⣬��ҪĿ���ǣ� ��
��1��2SO2 (g)��O2 (g)  2SO3 (g)��2�֣���
2SO3 (g)��2�֣���
SO2 ��V2O5�� SO3 ��2VO2��2�֣��� O2 ��4VO2�� 2V2O5��2�֣���
��2�� a��c��2�֣���
��3��SO3 (g)��H2O(l) �� H2SO4 (l)����H����130.3kJ/mol��2�֣���
��4���õ��ϸ�Ũ�ȵ�SO2 ��ԭ��ѭ�������ã�2�֣���
 2SO3 (g)��2�֣���
2SO3 (g)��2�֣���SO2 ��V2O5�� SO3 ��2VO2��2�֣��� O2 ��4VO2�� 2V2O5��2�֣���
��2�� a��c��2�֣���
��3��SO3 (g)��H2O(l) �� H2SO4 (l)����H����130.3kJ/mol��2�֣���
��4���õ��ϸ�Ũ�ȵ�SO2 ��ԭ��ѭ�������ã�2�֣���
��

��ϰ��ϵ�д�
�����Ŀ
 N2(g)+3H2(g)
N2(g)+3H2(g)  2NH3(g)����H= ��92.4kJ/mol
2NH3(g)����H= ��92.4kJ/mol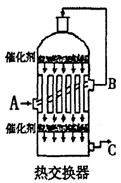
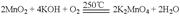 ��
��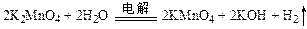 ��
��