��Ŀ����
��9�֣�ijͬѧ̽��ͬ����Ԫ�����ʵĵݱ���ɣ�������Ӱ�컯ѧ��Ӧ���ʵ����أ�ѡ�õ��Լ����£�þ�������������ۡ��ơ����Ƶ�Na2S��Һ�����Ƶ���ˮ��0.5mol/L�����ᡢ3mol/L�����ᡢ��̪��Һ������Ƶ�ʵ�鷽��������ʵ���������±���
��ش��������⣺
��1��ʵ��۵������� ���÷�Ӧ�����ӷ���ʽΪ ��
��2����ʵ��۵ó���ʵ�������
��3����ʵ��ڿɵó�������ѧ��Ӧ��������Ҫ������
��4��ʵ����У���Ϊ������ʧ���õ�ʱ��̣���ˣ���ͬѧ�ó����ۣ�����þ��ʧ���ӣ��ý����Ƿ���ȷ�� �����ǻ��
��5��ͨ��ʵ���˵��Ҫ�ӿ컯ѧ��Ӧ���ʿ� �� ��
| ʵ�鲽�� | ʵ������ |
| �ٽ�һС������Ʒ�����з�̪��Һ����ˮ�� | �ƿ鸡��ˮ���ϣ��ۻ���������С�����������ƶ�����֮��ʧ����Һ���ɫ |
| �ڽ������������ͬ��þ����������������ɰֽ��ĥ�����ֱ�Ͷ����������ͬ�����0.5mol/L�������� | þ�����ҷ�Ӧ��Ѹ�ٲ�����������ɫ���壬��������Ӧ��ʮ�־��ң�������ɫ���壬þ����ʧ�������� |
| �۽����Ƶ���ˮ�μӵ����Ƶ�Na2S��Һ�� | |
| �ܽ���ͬ������þ��������ɰֽ��ĥ���������۷ֱ�Ͷ�뵽��������ͬ�����0.5mol/L�������3mol/L�������� | ���ҷ�Ӧ�������壬��������ʧ��þ���� |
��ش��������⣺
��1��ʵ��۵������� ���÷�Ӧ�����ӷ���ʽΪ ��
��2����ʵ��۵ó���ʵ�������
��3����ʵ��ڿɵó�������ѧ��Ӧ��������Ҫ������
��4��ʵ����У���Ϊ������ʧ���õ�ʱ��̣���ˣ���ͬѧ�ó����ۣ�����þ��ʧ���ӣ��ý����Ƿ���ȷ�� �����ǻ��
��5��ͨ��ʵ���˵��Ҫ�ӿ컯ѧ��Ӧ���ʿ� �� ��
��1�����ɻ�ɫ���� Cl2 + S2�� = 2Cl��+ S����2�֣�
��2����ԭ�ӵõ�������ǿ����ԭ�ӵõ�������������������ͬ���÷֣���2�֣�
��3����Ӧ�ﱾ��������
��4����
��5������Ӧ��Ũ�� ����Ӧ��Ӵ�����������ע�������⣬ÿ��1�֣�
��2����ԭ�ӵõ�������ǿ����ԭ�ӵõ�������������������ͬ���÷֣���2�֣�
��3����Ӧ�ﱾ��������
��4����
��5������Ӧ��Ũ�� ����Ӧ��Ӵ�����������ע�������⣬ÿ��1�֣�
��

��ϰ��ϵ�д�
 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
�����Ŀ
 2SO3��g�� ��H����196.6 kJ��mol-1
2SO3��g�� ��H����196.6 kJ��mol-1
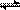 2Z ���ﵽƽ��� X��ת���ʲ�����Ϊ
2Z ���ﵽƽ��� X��ת���ʲ�����Ϊ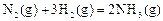

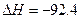 kJ��mol
kJ��mol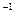 ��
��



