��Ŀ����
16��ϩ���е�̼̼˫����һ�������������������������ѣ�����ȩ��ͪ����CH3CH�TCH2$��_{����}^{O_{3}}$CH3CHO+HCHO
��CH3��2C�TCHCH3$��_{����}^{O_{3}}$CH3COCH3+CH3CHO��
�ش��������⣺
ij��ϩ��A�����������·�����Ӧ���õ����ֲ���B��C�Ļ�ѧʽ��ΪC3H6O������B�ܷ���������Ӧ��C���ܣ�д��A��B��C�Ľṹ��ʽ��
A��CH3��2C�TCHCH2CH3��BCH3CH2CHO��CCH3COCH3��
���� ij��ϩ��A��������Ϣ�������·�����Ӧ���õ����ֲ���B��C����B��CӦΪȩ��ͪ������B�ܷ���������Ӧ��C���ܣ���˵��B����ȩ����CΪͪ��B��C�Ļ�ѧʽ��ΪC3H6O����BΪCH3CH2CHO��CΪCH3COCH3�����������Ϣ����֪AΪ��CH3��2C�TCHCH2CH3���ݴ˴��⣮
��� �⣺ij��ϩ��A��������Ϣ�������·�����Ӧ���õ����ֲ���B��C����B��CӦΪȩ��ͪ������B�ܷ���������Ӧ��C���ܣ���˵��B����ȩ����CΪͪ��B��C�Ļ�ѧʽ��ΪC3H6O����BΪCH3CH2CHO��CΪCH3COCH3�����������Ϣ����֪AΪ��CH3��2C�TCHCH2CH3��
�ʴ�Ϊ����CH3��2C�TCHCH2CH3��CH3CH2CHO��CH3COCH3��
���� ���⿼���л�����ƶϣ��ѶȲ����� �Ĺؼ��dz������������Ϣ����Ϸ���ʽ�����ƶϣ�ע�ض��л�����֪ʶ�Ŀ��飬ͬʱ����ѧ��Ӧ����Ϣ�Լ���ϢǨ��������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
6��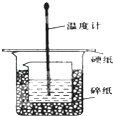 ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ���� ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺
ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ���� ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺
��1����ʵ���л�ȱ��һ���������������ǻ��β�����������ڴ�С�ձ��������ĭ���ϵ������DZ��¸��ȣ���ֹ����ɢʧ��
��2����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50mL������¼��ԭʼ���ݣ���������
��֪���ᡢNaOH��Һ�ܶȽ���Ϊ1.00g/cm3���кͺ���Һ�ı�����Ϊ��c=4.18��10-3kJ/��g•�棩����д���÷�Ӧ���Ȼ�ѧ����ʽ�����ӷ���ʽ��H+��aq��+OH-��aq��=H2O��l����H=-56.0kJ/mol ����Hֵ������С�����1λ����
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ�ȣ�2�������Hƫ���ƫ����ƫС�����䡱����
��4�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����������¶ȼƲⶨNaOH��Һ�¶ȵIJ��裬���˲������裬���õ��к��ȡ�H��ƫ���ƫ����ƫС�����䡱����
 ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ���� ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺
ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ���� ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺��1����ʵ���л�ȱ��һ���������������ǻ��β�����������ڴ�С�ձ��������ĭ���ϵ������DZ��¸��ȣ���ֹ����ɢʧ��
��2����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50mL������¼��ԭʼ���ݣ���������
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶ȣ�t2��/�� | �²� ��t2-t1��/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ�ȣ�2�������Hƫ���ƫ����ƫС�����䡱����
��4�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����������¶ȼƲⶨNaOH��Һ�¶ȵIJ��裬���˲������裬���õ��к��ȡ�H��ƫ���ƫ����ƫС�����䡱����
7������˵����ȷ���ǣ�������
| A�� | CuSO4 •5H2O�����������ﶼ�Ǵ����� | |
| B�� | һ�����ʲ��ǵ���ʾ��Ƿǵ���� | |
| C�� | ϡ���ᡢNaCl��Һ��ʵ���ҳ����ĵ���� | |
| D�� | ��������ɹ㷺����ʳƷ������ |
11������ȩ���Ҵ�����������������ɵĻ����0.1mol����ȫȼ�պ�����������ͨ��Ũ���ᣬȻ����ͨ��ʢ�м�ʯ�ҵĸ���ܣ����Ũ���������������5.4�ˣ���ԭ���������ȩ���Ҵ������������ʵ���֮��Ϊ��������
| A�� | 2��3��1 | B�� | 1��2��3 | C�� | 4��3��2 | D�� | 3��2��1 |
1��MgCl2•nH2O�����ڡ��㶹������ȡ2.03g�˽ᾧˮ��������ˮ������������AgNO3��Һ���õ��Ȼ�������2.87g����n��ֵ�ǣ�������
| A�� | 4 | B�� | 6 | C�� | 8 | D�� | 10 |
8���ء���Ϊͬ��Ԫ�أ��������ƣ��ֽ�һ�������Ͻ���ȫ�����ռ���Һ�еõ���ҺX����֪��
���������صĵ��뷽��ʽ��H++H2O+GaO2-?Ga��OH��3?Ga3++3OH-��
�������ռ���Һ��Ӧ�����ӷ���ʽ��2Ga+2H2O+2OH-=2GaO2-+3H2����
����X��Һ�л���ͨ��CO2����������������������Al��OH��3��
| Al��OH��3 | Ga��OH��3 | |
| ��ʽ���볣��Ka | 2��10-11 | 1��10-7 |
| ��ʽ���볣��Kb | 1.3��10-33 | 1.4��10-34 |
�������ռ���Һ��Ӧ�����ӷ���ʽ��2Ga+2H2O+2OH-=2GaO2-+3H2����
����X��Һ�л���ͨ��CO2����������������������Al��OH��3��
5���ں��º��������£���������������ֱ���ƽ�⣬����Ϊ��Чƽ����Ǣ٢ڢۣ�
| N2��g�� | + | 3H2��g�� | ? | 2NH3 | |
| �� | 1mol | 3mol | 0mol | ||
| �� | 0mol | 0mol | 2mol | ||
| �� | 0.5mol | 1.5mol | 1mol | ||
| �� | 1mol | 3mol | 2mol |
6��һ���¶��£�10mL 6mol/L��H2SO4��Һ�������Zn�۷�Ӧ��Ϊ�˼�����Ӧ���ʣ�����Ӱ������H2��������������Ӧ����ϵ�м��������ģ�������
| A�� | Na2CO3���� | B�� | CH3COONa���� | C�� | Na2SO4��Һ | D�� | NaNO3��Һ |