��Ŀ����
4��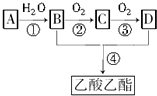 ��֪����A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ��
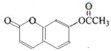
��֪����A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����2CH3CHO+O2$\frac{\underline{����}}{��}$2CH3COOH������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ���ش��������⣺
��1��A�Ŀռ乹����ƽ���Σ�
��2��A��C�����еĹ��������Ʒֱ���̼̼˫����ȩ����
��3��д�����з�Ӧ�ķ�Ӧ���ͣ��ټӳɷ�Ӧ��������Ӧ��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
��CH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH��
��2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O��
��CH3COOH+HOC2H5$��_{��}^{Ũ����}$CH3COOC2H5+H2O��
���� A��ʯ���ѽ�������Ҫ�ɷ֣������ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪCH2=CH2����ˮ�����ӳɷ�Ӧ�õ�BΪCH3CH2OH��B�������õ�CΪCH3CHO��C�����õ�DΪCH3COOH���Ҵ������ᷢ��������Ӧ�õ�EΪCH3COOCH2CH3���ݴ˽��
��� �⣺A��ʯ���ѽ�������Ҫ�ɷ֣������ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪCH2=CH2����ˮ�����ӳɷ�Ӧ�õ�BΪCH3CH2OH��B�������õ�CΪCH3CHO��C�����õ�DΪCH3COOH���Ҵ������ᷢ��������Ӧ�õ�EΪCH3COOCH2CH3��
��1��AΪCH2=CH2���ռ乹�ͣ�ƽ���Σ�
�ʴ�Ϊ��ƽ���Σ�
��2��AΪCH2=CH2�������еĹ�������̼̼˫����CΪCH3CHO�������еĹ�������ȩ����
�ʴ�Ϊ��̼̼˫����ȩ����
��3����Ӧ������ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����Ӧ�����Ҵ�����������Ӧ������ȩ��
�ʴ�Ϊ���ӳɷ�Ӧ��������Ӧ��
��4����Ӧ�ٵĻ�ѧ����ʽΪ��CH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH��
��Ӧ�ڵĻ�ѧ����ʽ��2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O��
��Ӧ�����������Ҵ�����������Ӧ����������������Ӧ����ʽΪ��CH3COOH+HOC2H5$��_{��}^{Ũ����}$CH3COOC2H5+H2O��
�ʴ�Ϊ��CH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH��2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O��CH3COOH+HOC2H5$��_{��}^{Ũ����}$CH3COOC2H5+H2O��
���� ���⿼���л�����ƶϣ��漰ϩ��������ȩ�������������ת������Ŀ�ѶȲ����ضԻ���֪ʶ�Ĺ��̣�

 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�| A�� | ��Ӧ����Һ�в������κγ��������Է�Ӧǰ��Cu2+��Ũ�Ȳ��� | |
| B�� | �����ܽ����������ɫ���������[Cu��NH3��4]2+ | |
| C�� | [Cu��NH3��4]2+�Ŀռ乹��Ϊ���������� | |
| D�� | ��[Cu��NH3��4]2+�����У�Cu2+�����¶Ե��ӣ�NH3�ṩ�չ�� |
| A�� | CH3CHO��C2H5OH | B�� | C2H5OH��CH2=CH2 | ||
| C�� | 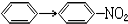 | D�� | CH3COOH��CH3COOC2H5 |
| A�� | ��������NaOH����Һ���� | B�� | �ױ���Ũ�����Ũ����ķ�Ӧ | ||
| C�� | ��������NaOHˮ��Һ���� | D�� | �����������ڹ��յ������·�Ӧ |
| A�� | Cl-���ӵĽṹʾ��ͼ�� | B�� | �����ӵı���ģ�ͣ� | ||
| C�� | H2O2�Ľṹʽ��H-O-O-H | D�� | CCl4�ĵ���ʽ��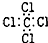 |
 ��
�� ��
��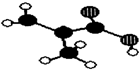 �㽭����������������ij��Ʒֻ��C��H��O����Ԫ�أ������ģ����ͼ��ʾ��ͼ��������֮������ߴ�����ѧ�����絥����˫���ȣ���
�㽭����������������ij��Ʒֻ��C��H��O����Ԫ�أ������ģ����ͼ��ʾ��ͼ��������֮������ߴ�����ѧ�����絥����˫���ȣ���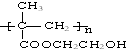 ��
�� ��ҵ�Ϻ;������ˮú���ڴ��������£���ˮ����������Ӧ��ȡ��������ѧ����ʽΪ��CO��g��+H2O��g��?H2��g��+CO2��g����һ�������£���4molCO��2molH2O��g���������Ϊ2L���ܱ������У���ϵ�и����ʵ������ʱ��仯��ͼ��ʾ��
��ҵ�Ϻ;������ˮú���ڴ��������£���ˮ����������Ӧ��ȡ��������ѧ����ʽΪ��CO��g��+H2O��g��?H2��g��+CO2��g����һ�������£���4molCO��2molH2O��g���������Ϊ2L���ܱ������У���ϵ�и����ʵ������ʱ��仯��ͼ��ʾ��