��Ŀ����
12����1�������£�0.1mol/L�������pH=1����2�������£�ij������Һ��pH=1����Ũ�ȣ�0.1mol/L�����������������=������
��3���͵�AgCl��Һ�м���NaBr��aq�����е���ɫ�������ɣ�˵��Ksp��AgCl����Ksp��AgBr�������������������=������
��4��Ba��OH��2��һ��ǿ����ʣ�����25�桢pH=13��Ba��OH��2��Һ����Һ����ˮ�����c��OH-��=10-13 mol•L-1��
��5��25�桢pH=13��Ba��OH��2��Һ��ijŨ��������Һ������ȣ�������֮�ȣ�1��9��Ϻ�������ҺpH=11����������Һ��������ڻ��ǰ����Һ������ͣ�����������Һ��pH=2��
��6�������£�ȡpH=2������ʹ�����Һ��100mL�������зֱ����������Zn������Ӧ����ʱ����Һ��pH��Ϊ4���������м����Zn����Ϊm1��������Һ�м����Zn����Ϊm2����m1��m2��ѡ���������=����������
���� ��1��pH=-lgc��H+�������㣻
��2��������������ʣ�������ȫ���룻
��3����ѧʽ���Ƶ����ʵ��ܶȻ�����ԽС��������Խ���ܣ����ݳ�����ת���ĽǶȷ�����
��4��pH=13��Ba��OH��2��Һ�������Ӷ�����ˮ���������ˮ���������c��H+��������Һ��ˮ�����c��OH-����
��5������������ʵ���Ũ��Ϊ��C��HCl��������������ֱ�Ϊ1L��9L���ֱ���������������Ӻ������ӵ����ʵ��������߷�������кͣ�������ҺpH=11��֪��ʣ�࣬���ʣ������������ӵ����ʵ��������ݣ�Kw=c��H+����c��OH -��������
��6����Ӧǰ�ͷ�Ӧ��Ԫ�غʹ�����Һ��������Ũ����ȣ����ݴ���Ϊ������ʡ�����Ϊǿ����ʷ�������п��������С��
��� �⣺��1������Ϊǿ�ᣬ0.1mol/L�����ᣬPH=-lgc��H+��=-lg0.1=1��
�ʴ�Ϊ��1����
��2�������£�ij������Һ��pH=1������PH=-lgc��H+����c��H+��=0.1mol/L������Ϊ������ʣ�����Һ�в��ֵ��룬��Ũ�ȴ���0.1mol/L��
�ʴ�Ϊ������
��3����AgCl������Һ�м�������ŨNaBr��Һ������AgBr������˵��AgBr��AgCl�����ܣ�˵��Ksp��AgCl����Ksp��AgBr����
�ʴ�Ϊ����
��4����pH=13��Ba��OH��2��Һ����������ˮ���������ˮ���������c��H+��������Һ��ˮ�����c��OH -������ˮ�����c��OH -��=c��H+��=10-13mol/L��
�ʴ�Ϊ��10-13 mol•L-1��
��5������������ʵ���Ũ��Ϊ��C��HCl��������������ֱ�Ϊ1L��9L����n��OH -��=0.1mol/L��1L=0.1mol��n��H+��=C��HCl����9L����Ӧ��ʣ�����������ӵ����ʵ���Ϊ��0.1mol-c��HCl����9L���л��������ҺpH=11����֪��Ϻ�c��H+��=10-11 mol•L-1�����Ϻ�c��OH -��=$\frac{{K}_{w}}{C��{H}^{+}��}$=10-3 mol•L-1��
����$\frac{0.1mol/L-C��HCl����9L}{10L}$=10-3 mol•L-1����ã�c��HCl��=10-2mol•L-1��pH=-lg[H+]������pH=2��
�ʴ�Ϊ��2
��6������ʹ����pH�仯������2��4��������������Ũ����С���������д��ڵ���ƽ�⣬�����Ӻ�п��Ӧʱ�ٽ�������룬���䷴Ӧ�������ӣ����Դ����DZ߷�Ӧ�ߵ���H+������Ӧ����Һ��pH��ȣ���������Ũ����ȣ����Է�Ӧ�����д������ĵ�Zn�࣬��������п������m1��m2��
�ʴ�Ϊ������
���� ���⿼������Һ���������ҺpH�ļ��㡢������ʵĵ���ƽ���֪ʶ����Ŀ�Ѷ��еȣ�ע��������Һ���������ҺpH�ļ��㷽������ȷ�����������Һ�в��ֵ�����ص㣮

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�| A�� | 2 molKClO3 | B�� | 22.4LHCl���� | C�� | 2 molNaCl | D�� | 1 mol AlCl3 |
| A�� | ������ | B�� | ��ɫ | C�� | ԭ���� | D�� | �ܶ� |
| A�� | 2H2S+SO2�T3S��+2H2O | |
| B�� | AlCl3+3NaAlO2+6H2O�T4Al��OH��3��+3NaCl | |
| C�� | 2NaBr+Cl2�T2NaCl+Br2 | |
| D�� | 2FeCl3+H2S�T2FeCl2+2HCl+S�� |

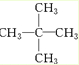 ������Ϊ2��2-�������飨�����飩��
������Ϊ2��2-�������飨�����飩��