��Ŀ����
4��������ؼ����Զ������̵ķ���֮һ�����õ���������ࣨ��Ҫ��MnO2��MnO����������Ǧ���ƵĻ�����ȣ���ԭ�ϲ��������¹����Ʊ���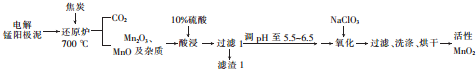
��1����ԭ¯�м��뽹̿����Mn2O3�Ļ�ѧ����ʽΪ4MnO2+C$\frac{\underline{\;����\;}}{\;}$2Mn2O3+CO2�������ʱ��Ϊ��߽�ȡ�ʿɲ��õĴ�ʩ���ӳ���ȡʱ�䣨��ֽ��衢��߽�ȡ�¶ȵȣ�����һ������
��2�����ˢ������������Ҫ�ɷ�ΪCaSO4��PbSO4���ѧʽ����
��3������pH��Mn2��SO4��3���绯������MnO2��MnSO4���ѧʽ��������NaClO3�Ȼ�Mn2+��Ӧ�����ӷ���ʽΪ3Mn2++ClO3-+3H2O=3MnO2��+6H++Cl-��
��4���ù����в�ֱ�������̿���Ҫ�ɷ�ΪMnO2����ԭ�϶����õ���̺������࣬���ŵ����������Դ�������ʣ����ֹ�̵Ĺ��������Ի��������Ⱦ�������о�һ�㣩��
��5����������������к���Ԫ�ص���������Ϊa%����ת����Ϊb%����1t�����������ɵõ�����MnO2�����ʵ���Ϊ$\frac{100ab}{55}$mol���ú���a��b�Ĵ���ʽ��ʾ����
���� �������������뽹̿��700���ڻ�ԭ¯�г�ַ�Ӧ���ɶ�����̼��Mn2O3��MnO�����ʣ�����10%�����������������Mn2��SO4��3��MnSO4�ȣ���������CaSO4��PbSO4�ȣ�����pH��5.5��6.5������NaClO3������������MnO2�������ˡ�ϴ�ӡ���ɵõ�����MnO2��
��1����ԭ¯�м��뽹̿����Mn2O3��ͬʱ���ɶ�����̼���壬���ʱ��Ϊ��߽�ȡ�ʣ��ɳ�ֽ��裬����Ӵ���������ʵ���߽�ȡ�¶ȣ����ӳ���ȡʱ�䣻
��2�������Ϸ�����֪��������CaSO4��PbSO4�ȣ��ʴ�Ϊ��CaSO4��PbSO4��
��3��Mn2��SO4��3��MnԪ�ػ��ϼ�Ϊ+3�ۣ���pH��5.5��6.5ʱ�����绯��Ӧ����MnO2��MnSO4������NaClO3������������MnO2��
��4�����õ���̺������࣬���������Դ�������ʣ����ٻ�����Ⱦ����ֹ�̵Ĺ��������Ի��������Ⱦ��
��5��������������к���Ԫ�ص���������Ϊa%����1t����������ຬ��MnԪ�ص�����Ϊ1��106g��a%�����ʵ���Ϊ$\frac{1{0}^{4}a}{55}$mol�����ת���ʼ��㣮
��� �⣺�������������뽹̿��700���ڻ�ԭ¯�г�ַ�Ӧ���ɶ�����̼��Mn2O3��MnO�����ʣ�����10%�����������������Mn2��SO4��3��MnSO4�ȣ���������CaSO4��PbSO4�ȣ�����pH��5.5��6.5������NaClO3������������MnO2�������ˡ�ϴ�ӡ���ɵõ�����MnO2��
��1����ԭ¯�м��뽹̿����Mn2O3��ͬʱ���ɶ�����̼���壬��Ӧ�ķ���ʽΪ4MnO2+C$\frac{\underline{\;����\;}}{\;}$2Mn2O3+CO2�������ʱ��Ϊ��߽�ȡ�ʣ��ɳ�ֽ��裬����Ӵ���������ʵ���߽�ȡ�¶ȣ����ӳ���ȡʱ�䣬
�ʴ�Ϊ��4MnO2+C$\frac{\underline{\;����\;}}{\;}$2Mn2O3+CO2�����ӳ���ȡʱ�䣨��ֽ��衢��߽�ȡ�¶ȵȣ���
��2�������Ϸ�����֪��������CaSO4��PbSO4�ȣ��ʴ�Ϊ��CaSO4��PbSO4��
��3��Mn2��SO4��3��MnԪ�ػ��ϼ�Ϊ+3�ۣ���pH��5.5��6.5ʱ�����绯��Ӧ����MnO2��MnSO4������NaClO3������������MnO2����Ӧ�����ӷ���ʽΪ3Mn2++ClO3-+3H2O=3MnO2��+6H++Cl-��
�ʴ�Ϊ��MnO2��MnSO4��3Mn2++ClO3-+3H2O=3MnO2��+6H++Cl-��
��4�����õ���̺������࣬���������Դ�������ʣ����ٻ�����Ⱦ����ֹ�̵Ĺ��������Ի��������Ⱦ��
�ʴ�Ϊ���������Դ�������ʣ����ֹ�̵Ĺ��������Ի��������Ⱦ����
��5��������������к���Ԫ�ص���������Ϊa%����1t����������ຬ��MnԪ�ص�����Ϊ1��106g��a%�����ʵ���Ϊ$\frac{1{0}^{4}a}{55}$mol����ת����Ϊb%��
������MnO2�����ʵ���Ϊ$\frac{1{0}^{4}a}{55}$mol��b%=$\frac{100ab}{55}$mol��
�ʴ�Ϊ��$\frac{100ab}{55}$��
���� ���⿼�����ʵ��Ʊ������ӣ��漰���ʵļ����Լ�������ת����֪ʶ��Ϊ��Ƶ���㣬��Ŀ��Ϊ�ۺϣ���һ���Ѷȣ�����ע��Ҫ��������������ݣ�����ѧ���ķ���������

| A�� | ��������ΪNA��NO2��CO2��������к��е���ԭ����Ϊ2NA | |
| B�� | 28g��ϩ��C2H4���ͻ����飨C4H8���Ļ�������к��е�̼ԭ����Ϊ2NA | |
| C�� | ���³�ѹ�£�92g��NO2��N2O4������庬�е�ԭ����Ϊ6NA | |
| D�� | ���³�ѹ�£�22.4L����������þ�۳�ַ�Ӧ��ת�Ƶĵ�����Ϊ2NA |
| A�� | ���ܽ�����ݣ����ж����Mg��HCO3��2��Һ���ò�����Mg��OH��2����MgCO3 | |
| B�� | �÷е����ݣ����Ʋ��ܷ�һЩҺ�������÷���ķ������뿪���Ŀ����� | |
| C�� | �÷�Ӧ�����ݵĴ�С�����жϲ�ͬ��Ӧ�ķ�Ӧ���ʵĿ��� | |
| D�� | ��ԭ�Ӱ뾶���ݣ����ƶ�ijЩԭ�������Ի�ԭ�Ե�ǿ�� |
| A�� | һ�����߷ֱ�ͨ����Һ�ͽ���ʱ�������ܿ���һ�������ġ�ͨ·����ǰ����û�� | |
| B�� | ���ǡ����ᱵ�Ͱ����ֱ����ڷǵ���ʡ�ǿ����ʺ�������� | |
| C�� | Mg��Al��Cu���Էֱ����û�����ֱ�Ӽ��ȷ��͵�ⷨұ���õ� | |
| D�� | ��������Ȼ����ˮú���ֱ����ڻ�ʯ��Դ����������Դ�Ͷ�����Դ |
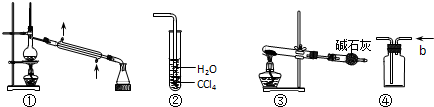
| A�� | װ�âٳ����ڷ���ijЩ���ܵ�Һ������ | |
| B�� | װ�âڿ���������HCl���壬����ֹ���� | |
| C�� | ��NH4HCO3Ϊԭ�ϣ�װ�âۿ�����ʵ�����Ʊ�����NH3 | |
| D�� | װ�â�b�ڽ����������ſ������ռ�H2��NO������ |
 ���ʽṹ�����������ʣ��ش��������⣺
���ʽṹ�����������ʣ��ش��������⣺ ��������ԭ��N���ӻ���ʽ��sp3��
��������ԭ��N���ӻ���ʽ��sp3�� X��Y��Z��RΪǰ������Ԫ�أ���ԭ��������������XY2�Ǻ���ɫ���壻Z��̬ԭ�ӵ�M����K���������ȣ�R2+��3d�����9�����ӣ�
X��Y��Z��RΪǰ������Ԫ�أ���ԭ��������������XY2�Ǻ���ɫ���壻Z��̬ԭ�ӵ�M����K���������ȣ�R2+��3d�����9�����ӣ�
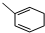
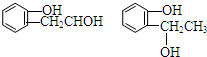 �ȣ�����FeCl3��Һ����ɫ�� ����Ũ������������ܷ�����ȥ��Ӧ��
�ȣ�����FeCl3��Һ����ɫ�� ����Ũ������������ܷ�����ȥ��Ӧ��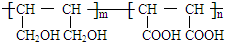 ��
�� ����CH2=CHCOOH�������Ʒ�Ӧ�ٵķ�Ӧ�����ɻ��������д������һ�ֽṹ��ʽΪ
����CH2=CHCOOH�������Ʒ�Ӧ�ٵķ�Ӧ�����ɻ��������д������һ�ֽṹ��ʽΪ ��
��