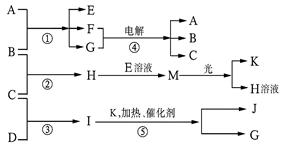
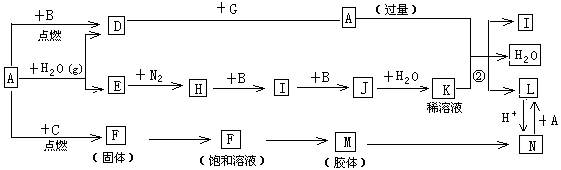
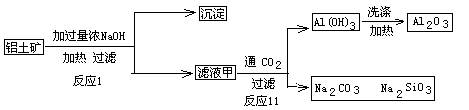
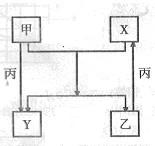
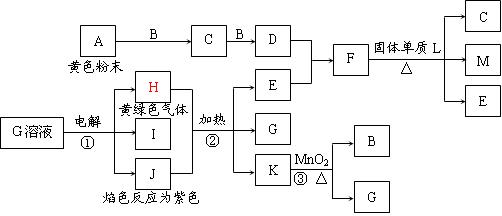
��Ŀ����
������Ԫ��W��X��Y��Z����Ԫ�����ڱ��е�λ����ͼ��ʾ��
����X��Y��Z����Ԫ�ص�������֮��Ϊ21��?
(1)W��Z�γ�ԭ�Ӹ�����Ϊ1��1�Ļ���������ʽΪ ��
(2)Y������������Ӧ��ˮ������Y���⻯��ǡ����ȫ��Ӧ���������ˮ��Һ��
���ԣ���ԭ���� (�û�ѧ�����ʾ)��
����Һ�и�������Ũ���ɴ�С��˳��Ϊ ��
(3)��X W4��Z2��KOH��Һ��ɵ�����ȼ�ϵ���У������Ϸ�����Ӧ�ĵ缫��ӦʽΪ
��
(4)��֪��2YZ2(g)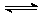 Y2Z4(g)����H��0���ں��º��������£���һ����XZ2��X2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��
Y2Z4(g)����H��0���ں��º��������£���һ����XZ2��X2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��
t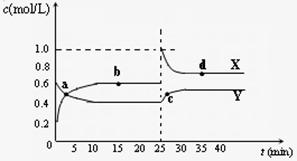 �ı仯��ϵ����ͼ��ʾ��
�ı仯��ϵ����ͼ��ʾ��
�� a��b��c��d�ĸ����У���ѧ��Ӧ
����ƽ��״̬���� �㡣
�� 25 minʱ��������
�������ʵĻ�ѧʽ�� mol��
�� a��b��c��d�ĸ���������ʾ�ķ�Ӧ��ϵ
�У�������ɫ���dz��˳���� __
������ĸ����
| W | | | | | | | |
| | | | X | Y | Z | | |
(1)W��Z�γ�ԭ�Ӹ�����Ϊ1��1�Ļ���������ʽΪ ��
(2)Y������������Ӧ��ˮ������Y���⻯��ǡ����ȫ��Ӧ���������ˮ��Һ��
���ԣ���ԭ���� (�û�ѧ�����ʾ)��
����Һ�и�������Ũ���ɴ�С��˳��Ϊ ��
(3)��X W4��Z2��KOH��Һ��ɵ�����ȼ�ϵ���У������Ϸ�����Ӧ�ĵ缫��ӦʽΪ
��
(4)��֪��2YZ2(g)
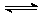 Y2Z4(g)����H��0���ں��º��������£���һ����XZ2��X2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��
Y2Z4(g)����H��0���ں��º��������£���һ����XZ2��X2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t
 �ı仯��ϵ����ͼ��ʾ��
�ı仯��ϵ����ͼ��ʾ���� a��b��c��d�ĸ����У���ѧ��Ӧ
����ƽ��״̬���� �㡣
�� 25 minʱ��������
�������ʵĻ�ѧʽ�� mol��
�� a��b��c��d�ĸ���������ʾ�ķ�Ӧ��ϵ
�У�������ɫ���dz��˳���� __
������ĸ����
(1) 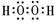 ��1�֣�
��1�֣�
��2��NH4+ + H2O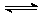 NH3��H2O + H+ ��1�֣�
NH3��H2O + H+ ��1�֣�
c (NO3-���� c (NH4+) �� c (H+) �� c (OH-) ��2�֣�
��3��CH4 - 8e- + 10OH -�� 4CO32- +7H2O ��2�֣�
��4����b,d��2�֣� ��NO2�� 0.8��2�֣� ��cdba ��2�֣�
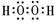 ��1�֣�
��1�֣���2��NH4+ + H2O
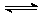 NH3��H2O + H+ ��1�֣�
NH3��H2O + H+ ��1�֣�c (NO3-���� c (NH4+) �� c (H+) �� c (OH-) ��2�֣�
��3��CH4 - 8e- + 10OH -�� 4CO32- +7H2O ��2�֣�
��4����b,d��2�֣� ��NO2�� 0.8��2�֣� ��cdba ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ