��Ŀ����
��15�֣���ͼ����ѧ��ѧ�������ʵ�ת����ϵ��ijЩ��Ӧ���������ֲ�������ȥ����A��GΪ�ճ������еij���������B��C��E��I��JΪ���壬����CΪ����ɫ���壬MΪ���ɫ���塣
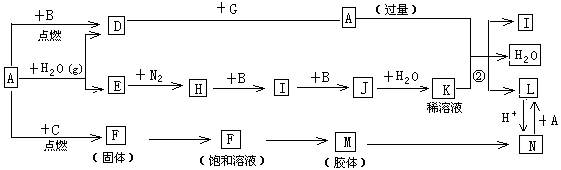
��1����֪AΪ��26��Ԫ�أ���д��AԪ�������ڱ��е�λ��__________��
��2��D��G��Ӧ�Ļ�ѧ����ʽ_________________________��
��3����Ӧ�����ӷ���ʽ__________________________��
��4��F��M�IJ�������_______________��
��5���ֽ�һ�Թ�J���嵹����ˮ����һ��ʱ���ˮ������������������ʹˮ���������Թܣ�Ӧ���Թ���ͨ��һ����__________��������Ļ�ѧʽ������ʱ�Թ�����Һ��Ũ��Ϊ________mol/L�������������״�����㣩��������λ��Ч���֣���
��6����X��Y��Ϊ������Ԫ�أ�X������������C��������Ԫ������������1���Ҳ�ͬ���ڣ�Y��Xͬ���壬���й���X��Y��C��Ԫ��˵����ȷ����__________��
A��ԭ�Ӱ뾶 X��Y��C B�����Ӱ뾶 X��C��Y
C���ǽ����� X��Y��C D������������Ӧˮ�������� C��X��Y
E���⻯��е� X��Y��C
����д��X���⻯����ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽ_________��
��7��D��G��Ӧ����A��ͬʱ��������һ�ֲ����ҵ������������Ҫ�ɷ�ΪAl2O3����SiO2��Fe2O3����ȡ��Ĺ����������£�
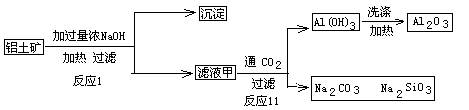
��Ϸ�ӦII���ж�������������ӣ�H+����������ǿ������˳����_________������ĸ��ţ���
A��AlO2�� B��OH�� C��SiO32��

��1����֪AΪ��26��Ԫ�أ���д��AԪ�������ڱ��е�λ��__________��
��2��D��G��Ӧ�Ļ�ѧ����ʽ_________________________��
��3����Ӧ�����ӷ���ʽ__________________________��
��4��F��M�IJ�������_______________��
��5���ֽ�һ�Թ�J���嵹����ˮ����һ��ʱ���ˮ������������������ʹˮ���������Թܣ�Ӧ���Թ���ͨ��һ����__________��������Ļ�ѧʽ������ʱ�Թ�����Һ��Ũ��Ϊ________mol/L�������������״�����㣩��������λ��Ч���֣���
��6����X��Y��Ϊ������Ԫ�أ�X������������C��������Ԫ������������1���Ҳ�ͬ���ڣ�Y��Xͬ���壬���й���X��Y��C��Ԫ��˵����ȷ����__________��
A��ԭ�Ӱ뾶 X��Y��C B�����Ӱ뾶 X��C��Y
C���ǽ����� X��Y��C D������������Ӧˮ�������� C��X��Y
E���⻯��е� X��Y��C
����д��X���⻯����ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽ_________��
��7��D��G��Ӧ����A��ͬʱ��������һ�ֲ����ҵ������������Ҫ�ɷ�ΪAl2O3����SiO2��Fe2O3����ȡ��Ĺ����������£�

��Ϸ�ӦII���ж�������������ӣ�H+����������ǿ������˳����_________������ĸ��ţ���
A��AlO2�� B��OH�� C��SiO32��
��1���������� ��VIII�� 1��
��2��8Ai + 3Fe3O4 =����= 4Al2O3 + 9Fe 2��
��3�� 3Fe + 8H+ + 2NO3�� ="==" 3Fe2+ + 2NO��+ 4H2O 2��
��4�������ȼ������һ������أ���һ��þ������ȼþ�� 2��
��5��O2 1�� 0.045 2��
��6��B 1��
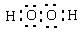 2��
2����7��BAC 2��
��

��ϰ��ϵ�д�
�����Ŀ
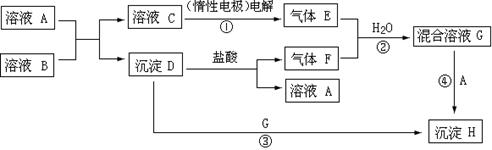
 : n(F)��1:1�����ڻ����ҺG�е��뼸��ʯ��
: n(F)��1:1�����ڻ����ҺG�е��뼸��ʯ��
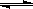 6H2O+5N2����һ�������£��÷�Ӧ�ﵽƽ��̬I�������¶�ƽ�ⷢ���ƶ����ﵽƽ��̬II�ķ�Ӧ������ʱ��仯�Ĺ�ϵͼ���ҡ��÷�ӦΪ ���� ������ȡ����ȡ�����Ӧ�������¶ȣ��÷�Ӧ��ƽ�ⳣ��Kֵ���������������������С�����䡱��
6H2O+5N2����һ�������£��÷�Ӧ�ﵽƽ��̬I�������¶�ƽ�ⷢ���ƶ����ﵽƽ��̬II�ķ�Ӧ������ʱ��仯�Ĺ�ϵͼ���ҡ��÷�ӦΪ ���� ������ȡ����ȡ�����Ӧ�������¶ȣ��÷�Ӧ��ƽ�ⳣ��Kֵ���������������������С�����䡱��

 Y2Z4(g)����H��0���ں��º��������£���һ����XZ2��X2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��
Y2Z4(g)����H��0���ں��º��������£���һ����XZ2��X2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��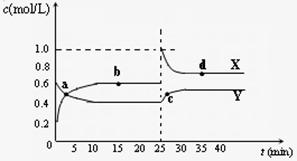 �ı仯��ϵ����ͼ��ʾ��
�ı仯��ϵ����ͼ��ʾ��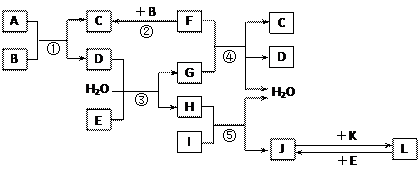
 ����ɫ�̣�D����+F����
����ɫ�̣�D����+F���� ��ɫ���壬A����+F����
��ɫ���壬A����+F����