��Ŀ����
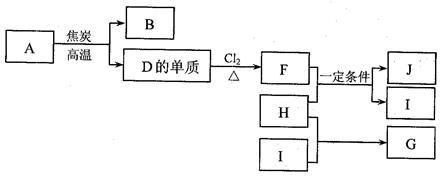
��11�֣�A��B��C��D����Ԫ�ض��Ƕ�����Ԫ�أ�AԪ�ص����Ӿ��л�ɫ����ɫ��Ӧ��A��BԪ�ص����ӽṹ��Ne������ͬ�ĵ��Ӳ��Ų���5.8 g B����������ǡ������100 mL 2 mol��L��1������ȫ��Ӧ��Bԭ�Ӻ�������������������ȡ�H2��C������ȼ�ղ�����ɫ���档 C������������ˮ���������к�������������ǿ�ģ�DԪ��ԭ�ӵĵ��Ӳ�ṹ�У������������Ǵ�����������3�����������������ش�
��1��Ԫ��Cλ�ڵ�__________���ڵ�__________�壬��������������ˮ����Ļ�ѧʽΪ____________��
��2��AԪ����_______��BԪ����______��DԪ����______����дԪ�ط��ţ�
��3��A��D�γɵ�ԭ�Ӹ�����Ϊ2:1�Ļ�����Ļ�ѧʽ��___ _ ____��
�õ���ʽ��ʾ�����γɹ���
��4��CԪ�صĵ����ж�������A������������Ӧ��ˮ�������Һ���գ�
�����ӷ���ʽΪ______________________________ __________________��
��1��Ԫ��Cλ�ڵ�__________���ڵ�__________�壬��������������ˮ����Ļ�ѧʽΪ____________��
��2��AԪ����_______��BԪ����______��DԪ����______����дԪ�ط��ţ�
��3��A��D�γɵ�ԭ�Ӹ�����Ϊ2:1�Ļ�����Ļ�ѧʽ��___ _ ____��
�õ���ʽ��ʾ�����γɹ���
��4��CԪ�صĵ����ж�������A������������Ӧ��ˮ�������Һ���գ�
�����ӷ���ʽΪ______________________________ __________________��
��1���� VIIA HClO4��2��Na Mg O ��3��Na2O ��
��4��Cl2 + 2OH- =" " Cl- + ClO - + H2O
��4��Cl2 + 2OH- =" " Cl- + ClO - + H2O
��

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ
 Y2Z4(g)����H��0���ں��º��������£���һ����XZ2��X2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��
Y2Z4(g)����H��0���ں��º��������£���һ����XZ2��X2Z4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��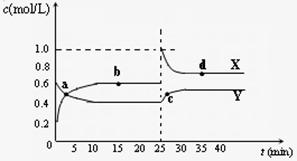 �ı仯��ϵ����ͼ��ʾ��
�ı仯��ϵ����ͼ��ʾ��