��Ŀ����
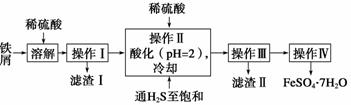
�̷�(FeSO4��7H2O)������ȱ����ƶѪҩƷ����Ҫ�ɷ֡���������������м(����������������������)Ϊԭ�����������̷���һ�ַ�����
��ѯ���ϣ����й����ʵ��������±���
| 25 ��ʱ | pHֵ |
| ����H2S��Һ | 3.9 |
| SnS������ȫ | 1.6 |
| FeS��ʼ���� | 3.0 |
| FeS������ȫ | 5.5 |
(1)�������У�ͨ�����������͵�Ŀ����____________������Һ���������ữ��pH��2��Ŀ����_______________________________________________________��
(2)��������˳������Ϊ����Ũ������ȴ�ᾧ��___________________________��
(3)�������õ����̷�������������ˮϴ�ӣ���Ŀ���ǣ��ٳ�ȥ������渽�ŵ���������ʣ���________________________________________________________��
(4)�ⶨ�̷���Ʒ��Fe2�������ķ����ǣ�a.��ȡ2.850 g�̷���Ʒ���ܽ⣬��250 mL����ƿ�ж��ݣ�b.��ȡ25.00 mL������Һ����ƿ�У�c.�������ữ��0.010 00 mol��L��1 KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00 mL��
�ٵζ�ʱʢ��KMnO4��Һ������Ϊ________(����������)��
���жϴ˵ζ�ʵ��ﵽ�յ�ķ�����_____________________________________��
�ۼ���������Ʒ��FeSO4��7H2O����������Ϊ_______________________________��
(1)��ȥ��Һ�е�Sn2��������ֹFe2���������� ��ֹFe2�����ɳ���
(2)����ϴ��
(3)����ϴ�ӹ�����FeSO4��7H2O�����
(4)����ʽ�ζ��ܡ��ڵμ����һ��KMnO4��Һʱ����Һ���dz��ɫ�Ұ�����ڲ���ɫ
��97.5%

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�ͨ����ʵ������Ĺ۲졢���������ó���ȷ�Ľ����ǻ�ѧѧϰ�ķ���֮һ��������ʵ����ʵ�Ľ�����ȷ����
| ���� | ���� | |
| A | KI������Һ��ͨ��Cl2����Һ���� | Cl2������۷�����ɫ��Ӧ |
| B | ŨHNO3�ڹ��������±�� | ŨHNO3���ȶ���������ɫ����NO2������Ũ���� |
| C | ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������� | ˵������Һ�к���SO |
| D | ͭƬ����Ũ�����У������Ա仯 | ˵��ͭ�����Ũ�����з����ۻ� |
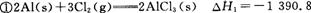 kJ/mol
kJ/mol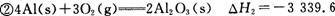 kJ/mol
kJ/mol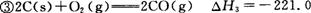 kJ/mol
kJ/mol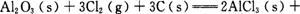
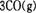 �Ħ�AH= ��
�Ħ�AH= ��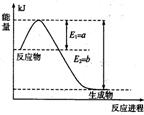 ���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á�
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á�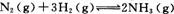 ��һ���¶��£���һ������N2��H2ͨ�뵽���Ϊ1 L���ܱ������У���Ӧ�ﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ���ƽ�ⳣ��������� ��
��һ���¶��£���һ������N2��H2ͨ�뵽���Ϊ1 L���ܱ������У���Ӧ�ﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ���ƽ�ⳣ��������� �� ����3 m01FeS2�μӷ�Ӧ��ת�� mol���ӡ�
����3 m01FeS2�μӷ�Ӧ��ת�� mol���ӡ�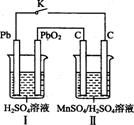 ��.�̼��仯����Ӧ��Խ��Խ�㷺��MnO2��һ����Ҫ�������ܲ��ϣ��Ʊ�Mn02�ķ���֮һ����ʯīΪ�缫������ữ��MnS04��Һ�������ĵ缫��ӦʽΪ ������Ǧ����Ϊ��Դ����ữ��MnS04��Һ����ͼ��ʾ��Ǧ���ص��ܷ�Ӧ����ʽΪ ������������4 mol H+������ʱ�����·��ͨ���ĵ��ӵ����ʵ���Ϊ ��MnO2�����۲���Ϊ g��
��.�̼��仯����Ӧ��Խ��Խ�㷺��MnO2��һ����Ҫ�������ܲ��ϣ��Ʊ�Mn02�ķ���֮һ����ʯīΪ�缫������ữ��MnS04��Һ�������ĵ缫��ӦʽΪ ������Ǧ����Ϊ��Դ����ữ��MnS04��Һ����ͼ��ʾ��Ǧ���ص��ܷ�Ӧ����ʽΪ ������������4 mol H+������ʱ�����·��ͨ���ĵ��ӵ����ʵ���Ϊ ��MnO2�����۲���Ϊ g��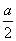 mol�����������ʵ�����0
mol�����������ʵ�����0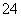
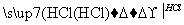 Y
Y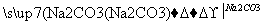 Z
Z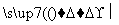 X
X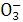 Ϊ(����)
Ϊ(����)