��Ŀ����
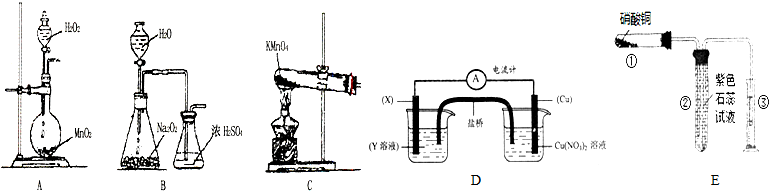
3�����Ȼ�����S2C12���ڹ�ҵ�ϳ�����������ʵ���ҿ�����ͼ1װ�ã���ȥ�г�������ģ�����ɹ��̣�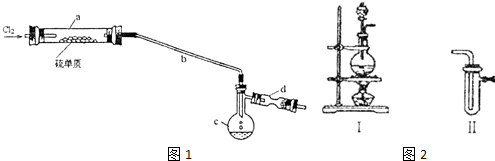
�������ϣ���S2Cl2��һ�ֽ��ɫ�ӷ�Һ�壨�۵㣺-76�棬�е㣺138�棩������ǿ�ҵ���Ϣ�ԣ�������ˮ����ˮ�ⷴӦ��
�ڽ������������110�桫140������Ӧ�����õ�S2C12��Ʒ��
��1����ͼ2װ�â����ӿ���ȡ�������������������������װ���ڵ��Լ������DZ���ʳ��ˮ��Ũ���ᣮ�Ʊ�C12�Ĺ����У�ijʵ��С�����ø����չ�����SO2��NaOH��Һ����C12����ֹ��Ⱦ����������һ��ʱ���ijͬѧȡ��2mL���պ����Һ��ǿ���ԣ����Թ��У�����������еμ�3��4�ε���-KI��Һ��������Һ�ȱ������漴����ȥ����Һ�ȱ�����˵������Cl2�����Һ�д���ClO-���������ţ�����ɫ��ȥ�Ŀ���ԭ����I2+2OH-=I-+IO-+H2O�������ӷ���ʽ�ش𣩣�
��2����������b�������ǵ���������
��3����ʵ��IJ���˳��ӦΪBADC ������ű�ʾ����
A������װ��a B��ͨ��C12 C��ֹͣͨCl2 D��ֹͣ����װ��a
��4��װ��d��Ӧ���õ��Լ�Ϊ��ʯ�ң�������Ϊ����β����������ֹ�����е�ˮ����ʹS2Cl2ˮ�⣮
���� ��1���������̺�Ũ������ȷ�Ӧ���������������к���ˮ�������Ȼ������壬����ͨ������ʳ��ˮ��ȥ�Ȼ��⣬ͨ��Ũ�����ȥˮ������ȡ��2mL���պ����Һ��ǿ���ԣ����Թ��У�����������еμ�3��4�ε���-KI��Һ��������Һ�ȱ�����˵���������е����ӵ����������ӣ��漴����ȥ����Һ�ȱ�����˵������Cl2�����Һ�д���Ϊ����������ӣ���ɫ��ȥ�ǵⵥ�ʺ�����������Һ��Ӧ���ɵ⻯�ơ��ε����ƺ�ˮ��
��2����������b�������ǵ����������ã�
��3������֮ǰ��ͨ����ˮ������ʼ���ɵ�S2Cl2������ȴҺ���������ֹͣ���Ⱥ�ֹͣͨ������ƽ��������ѹǿ����ֹ����Σ�գ�
��4��dװ��ʢ�ż�ʯ�ң�����Cl2β������ֹ�����е�ˮ������c�У�
��� �⣺��1��װ�â������ö������̺�Ũ������ȷ�Ӧ���������������к���ˮ�������Ȼ������壬��ͼ2װ�â����ӿ���ȡ�������������������������װ���ڵ��Լ������ǣ�����ͨ������ʳ��ˮ��ȥ�Ȼ��⣬ͨ��Ũ�����ȥˮ������ȡ��2mL���պ����Һ��ǿ���ԣ����Թ��У�����������еμ�3��4�ε���-KI��Һ��������Һ�ȱ�����˵���������е����ӵ����������ӣ��漴����ȥ����Һ�ȱ�����˵������Cl2�����Һ�д���Ϊ����������ӣ���ɫ��ȥ�ǵⵥ�ʺ�����������Һ��Ӧ���ɵ⻯�ơ��ε����ƺ�ˮ����Ӧ�����ӷ���ʽΪ��I2+2OH-=I-+IO-+H2O��
�ʴ�Ϊ������ʳ��ˮ��Ũ���ᣬClO-��I2+2OH-=I-+IO-+H2O��
��2���������������110�桫140������Ӧ�����õ�S2C12��Ʒ����������b�������ǵ���������
�ʴ�Ϊ������������
��3������֮ǰ��ͨ����ˮ������ʼ���ɵ�S2Cl2������ȴҺ���������ֹͣ���Ⱥ�ֹͣͨ������ƽ��������ѹǿ����ֹ����Σ�գ�����ʵ�����˳��Ϊ��BADC��
�ʴ�Ϊ��BADC��
��4��dװ��ʢ�ż�ʯ�ң�����Cl2β������ֹ��Ⱦ��������ֹ�����е�ˮ������c��ʹS2Cl2ˮ�⣬
�ʴ�Ϊ����ʯ�ң�����Cl2β������ֹ��Ⱦ��������ֹ�����е�ˮ����c��ʹS2Cl2ˮ�⣻
���� ���⿼��ѧ����ʵ��ԭ����װ�õ����⡢���ۣ��Ķ���Ŀ��ȡ��Ϣ�������ȣ��ؼ������������Ʊ�����ԭ�������������и�װ�õ����ã�Ҫ��ѧ��Ҫ����ʵ��ʵ�����֪ʶ�����Ӧ����Ϣ���������������Ѷ��еȣ�

 ʵʩ�Լ�����Դ�˷Ѻͽ��ͷ����ŷ�Ϊ�������ݵĽ��ܼ������ߣ���Ӧ��ȫ���������⡢������Դ��Լ�͡������Ѻ������ı�Ȼѡ������ҵ�ķ�չ������Ϲ��ҽ��ܼ��ŵ�����Ҫ����������ѧ֪ʶ������������⣺
ʵʩ�Լ�����Դ�˷Ѻͽ��ͷ����ŷ�Ϊ�������ݵĽ��ܼ������ߣ���Ӧ��ȫ���������⡢������Դ��Լ�͡������Ѻ������ı�Ȼѡ������ҵ�ķ�չ������Ϲ��ҽ��ܼ��ŵ�����Ҫ����������ѧ֪ʶ������������⣺��1����֪ij��Ӧ��ƽ�����ʽΪ��K=$\frac{{C��{H_2}��C��CO��}}{{C��{H_2}O��}}$����������ÿ����3gˮ������������22kJ����������÷�Ӧ���Ȼ�ѧ��ӦΪ��C��s��+H2O��g��
 CO��g��+H2��g����H=+132kJ/mol
CO��g��+H2��g����H=+132kJ/mol��2����֪��һ���¶��£�
C��s��+CO2��g��?2CO��g��ƽ�ⳣ��K1��
CO��g��+H2O��g��?H2��g��+CO2��g��ƽ�ⳣ��K2��
C��s��+H2O��g��?CO��g��+H2��g�� ƽ�ⳣ��K3��
��K1��K2��K3֮��Ĺ�ϵ�ǣ�K3=K1��K2��
��3��ú����ͨ��ͨ���о���ͬ�¶���ƽ�ⳣ���Խ������ʵ�����⣮��֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���ᷢ�����·�Ӧ��CO��g��+H2O��g��?H2��g��+CO2��g�����÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
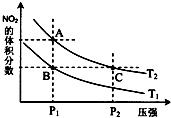
��4���Ӱ����������������ᣬ�˹������漰���������NO��NO2��N2O4�ȣ��Է�ӦN2O4��g��?2NO2��g����H��0���¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����D��
A��A��C����ķ�Ӧ���ʣ�A��C
B��A��C�����������ɫ��A�Cdz
C��B��C����������ƽ����Է���������B��C
D����״̬B��״̬A�������ü��ȵķ���
E��A��C����Ļ�ѧƽ�ⳣ����A��C
��5��0.2mol/L��NaOH��0.4mol/L���������Һ�������Ϻ���Һ�и����ӵ����ʵ���Ũ�ȴӴ�С��˳����c��NO3-����c��NH4+����c��Na+����c��OH-����c��H+����
 ��
�� ������ͬԪ�ص�ԭ�ӡ�
������ͬԪ�ص�ԭ�ӡ�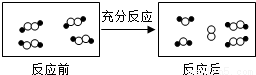

 ��
��