��Ŀ����
����ĺ�ˮ����Լ��1.4��1018 t��������������Դ��
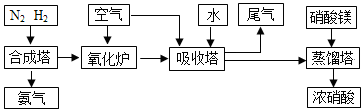
��1����ͼ���ú�ˮ�õ���ˮ�ķ���Ϊ________��
��2�����������ǽ��귢չ������һ�ֽϺõĺ�ˮ������������ԭ����ͼ��
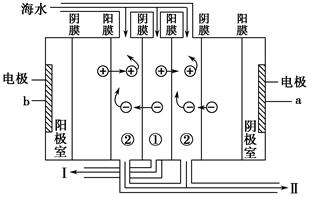
a�ǵ�Դ��________����I���ų�����________�����ˮ����Ũˮ������
��3����ˮ�������Ũˮ�к������η֣�����ˮ�л�ı�ˮ�ʣ��ŵ������лᵼ�������μ���ʲ���ֱ���ŷţ��������ȼҵ������
�ٵ��ǰ��Ҫ��Ũˮ���ƣ������Լ���˳��Ϊ___________________________________��
�������ӽ���Ĥ���۵��ʳ��ˮ�Ļ�ѧ����ʽΪ
_________________________________________________________________________________________________________________________________��
��4�����Ǻ˷�Ӧ����Ҫ��ȼ�ϣ�����������ֱ�ӹ�ϵ��һ�����Һ˹�ҵ��������ķ�չˮƽ����ˮ������UCl4��ʽ���ڣ�ÿ�ֺ�ˮֻ��3.3 mg�ˣ���ˮ���������������൱���ٹ�������̽����ˮ���˵ķ��������ڣ��Ѿ����Ƴɹ���һ�����������ӽ�����֬����ר��������ˮ�е��ˣ�������������Ԫ�ء��䷴Ӧԭ��Ϊ________����֬��HR���棩���������ӽ���������ӽ���Ĥ���ᴦ�������������õ����˵���Һ���䷴Ӧԭ��Ϊ_______________________________________________________________________________________________________________________________��
��1����ͼ���ú�ˮ�õ���ˮ�ķ���Ϊ________��

��2�����������ǽ��귢չ������һ�ֽϺõĺ�ˮ������������ԭ����ͼ��

a�ǵ�Դ��________����I���ų�����________�����ˮ����Ũˮ������
��3����ˮ�������Ũˮ�к������η֣�����ˮ�л�ı�ˮ�ʣ��ŵ������лᵼ�������μ���ʲ���ֱ���ŷţ��������ȼҵ������
�ٵ��ǰ��Ҫ��Ũˮ���ƣ������Լ���˳��Ϊ___________________________________��
�������ӽ���Ĥ���۵��ʳ��ˮ�Ļ�ѧ����ʽΪ
_________________________________________________________________________________________________________________________________��
��4�����Ǻ˷�Ӧ����Ҫ��ȼ�ϣ�����������ֱ�ӹ�ϵ��һ�����Һ˹�ҵ��������ķ�չˮƽ����ˮ������UCl4��ʽ���ڣ�ÿ�ֺ�ˮֻ��3.3 mg�ˣ���ˮ���������������൱���ٹ�������̽����ˮ���˵ķ��������ڣ��Ѿ����Ƴɹ���һ�����������ӽ�����֬����ר��������ˮ�е��ˣ�������������Ԫ�ء��䷴Ӧԭ��Ϊ________����֬��HR���棩���������ӽ���������ӽ���Ĥ���ᴦ�������������õ����˵���Һ���䷴Ӧԭ��Ϊ_______________________________________________________________________________________________________________________________��
��1������
��2��������ˮ
��3����NaOH��BaCl2��NaOH��BaCl2λ�ÿɻ�������Na2CO3������
��2NaCl��2H2O 2NaOH��H2����Cl2��
2NaOH��H2����Cl2��
��4��4HR��U4��=UR4��4H����UR4��4H��=4HR��U4��
��2��������ˮ
��3����NaOH��BaCl2��NaOH��BaCl2λ�ÿɻ�������Na2CO3������
��2NaCl��2H2O
 2NaOH��H2����Cl2��
2NaOH��H2����Cl2����4��4HR��U4��=UR4��4H����UR4��4H��=4HR��U4��
��1����ˮ���������������õ���ˮ��ԭ����������ͬ���ʷ����ɹ���Ϊ������2���������ӵ�Դ�����������ӵ�Դ�����Լ�ͼ�������������ƶ������֪a�ǵ�Դ�ĸ��������õ糡���ã�ʹ��ˮ�����������ӷֱ����������������ƶ���������Ĥ����Ĥ������ѡ�����������£�ʹ�еļ��������Ũ�ȱ����ڼ�������еļ��������Ũ�ȱ�С����ټ�������ʵó�����ų����ǵ�ˮ����3��Ũˮ�辫�ƺ���ܵ�⣬����ʱһ���ȼ��ռ��ȥMg2����Fe3���ȣ��ټ��Ȼ�����ȥSO42-���ټ�̼���Ƴ�ȥCa2��������Ba2�������˳���������������pH����4���������ӽ�����֬������Һ�������ӵ����ӷ���ʽ��Ǩ�Ƶ����������ӵ����ӷ���ʽ��һ��Ҫע�������Ӵ�4����λ������ɣ����ᴦ�����������֬�ֵõ������Ӻ���֬��

��ϰ��ϵ�д�
�����Ŀ

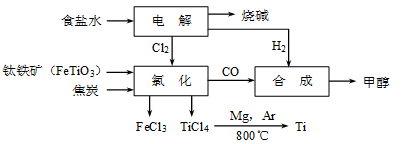
 CH3OH(g)�����������������������ʵ��κ���ʧ��������ҵ����ÿ�ϳ�6mol�״�����������ⲹ��H2 mol��
CH3OH(g)�����������������������ʵ��κ���ʧ��������ҵ����ÿ�ϳ�6mol�״�����������ⲹ��H2 mol��