��Ŀ����
���û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч��������������ij�������Ƹ﹤ҵ������Cr(��)�Ĵ��������������¡�
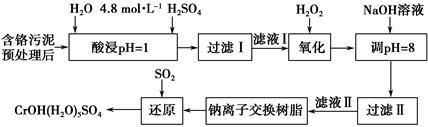
���������ȡҺ�еĽ���������Ҫ��Cr3���������Fe3����Al3����Ca2����Mg2����
(1)ʵ������18.4 mol��L��1��Ũ��������250 mL 4.8 mol��L��1��H2SO4��Һ�����õIJ����������ձ�������������Ͳ�⣬����_______________________ _��
(2)���ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ��_____________________________
______________________________________________________(�������)��
(3)H2O2�������ǽ���Һ���е�Cr3��ת��ΪCr2O72����д���˷�Ӧ�����ӷ���ʽ��_____________________________________________________________��
(4)�����£�����������������������ʽ����ʱ��Һ��pH���£�
����NaOH��Һʹ��Һ�ʼ��ԣ�Cr2O72��ת��ΪCrO42������Һ������������Ҫ��________������Һ��pH���ܳ���8����������_________________________��
(5)�����ӽ�����֬�ķ�Ӧԭ��ΪMn����nNaR�D��MRn��nNa�������������ӽ�����֬��ȥ����Һ���еĽ�����������_______________________________��
(6)д��������������SO2���л�ԭʱ������Ӧ�Ļ�ѧ����ʽ��__________________________________________________��

���������ȡҺ�еĽ���������Ҫ��Cr3���������Fe3����Al3����Ca2����Mg2����
(1)ʵ������18.4 mol��L��1��Ũ��������250 mL 4.8 mol��L��1��H2SO4��Һ�����õIJ����������ձ�������������Ͳ�⣬����_______________________ _��
(2)���ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ��_____________________________
______________________________________________________(�������)��
(3)H2O2�������ǽ���Һ���е�Cr3��ת��ΪCr2O72����д���˷�Ӧ�����ӷ���ʽ��_____________________________________________________________��
(4)�����£�����������������������ʽ����ʱ��Һ��pH���£�
| ������ | Fe3�� | Mg2�� | Al3�� | Cr3�� |
| ��ʼ����ʱ��pH | 2.7 | �� | �� | �� |
| ������ȫʱ��pH | 3.7 | 11.1 | 8 | 9(>9�ܽ�) |
����NaOH��Һʹ��Һ�ʼ��ԣ�Cr2O72��ת��ΪCrO42������Һ������������Ҫ��________������Һ��pH���ܳ���8����������_________________________��
(5)�����ӽ�����֬�ķ�Ӧԭ��ΪMn����nNaR�D��MRn��nNa�������������ӽ�����֬��ȥ����Һ���еĽ�����������_______________________________��
(6)д��������������SO2���л�ԭʱ������Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
��(1)250 mL����ƿ����ͷ�ι�
(2)���߷�Ӧ�¶ȡ������������ı�������ӿ�����ٶȵ�(�����㼴��)
(3)2Cr3����3H2O2��H2O===Cr2O72����8H��
(4)Na����Mg2����Ca2����pH����8��ʹ����Al(OH)3�ܽ�����AlO2��������Ӱ��Cr(��)�Ļ�����������
(5)Ca2����Mg2��
(6)3SO2��2Na2CrO4��12H2O===2CrOH(H2O)5SO4����Na2SO4��2NaOH
(2)���߷�Ӧ�¶ȡ������������ı�������ӿ�����ٶȵ�(�����㼴��)
(3)2Cr3����3H2O2��H2O===Cr2O72����8H��
(4)Na����Mg2����Ca2����pH����8��ʹ����Al(OH)3�ܽ�����AlO2��������Ӱ��Cr(��)�Ļ�����������
(5)Ca2����Mg2��
(6)3SO2��2Na2CrO4��12H2O===2CrOH(H2O)5SO4����Na2SO4��2NaOH
��(1)���һ�����ʵ���Ũ�ȵ���Һ�����Ʋ��輴��д��ȱ�ٵIJ���������ע������ƿ����ע�����(4)����NaOH��Һ����pH��8��Fe3����Al3����Fe(OH)3��Al(OH)3��ʽ������������Һ�л���Mg2����Ca2���������Na����(5)���������ӽ�����֬��Ŀ���dz�ȥ��Һ���е�Mg2����Ca2����

��ϰ��ϵ�д�
�����Ŀ