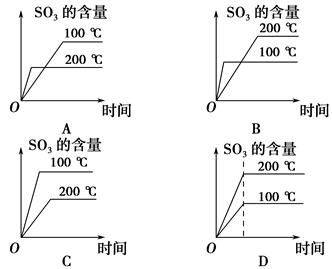
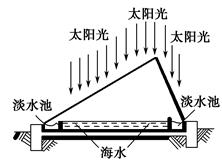
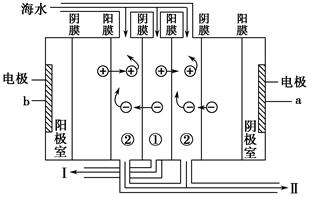
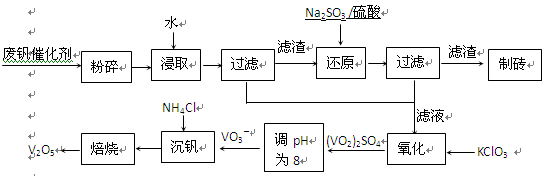
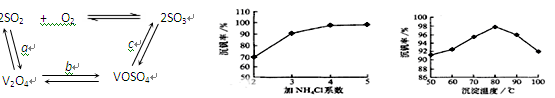
��Ŀ����
ij�ۺ�ʵ���С�鵽����ˮ�����вιۣ��˽Դˮ����������ˮ�Ĺ�������ʾ��ͼ���£�

��1��Դˮ�к�Ca2����Mg2����HCO3-��Cl���ȣ�����CaO������Ca��OH��2�������������ɸ����ֽⷴӦ����д����������һ����Ӧ�����ӷ���ʽ
_____________________________________________________________��
��2���������ۼ����Գ�ȥ���е���������������ù�����________����д���и������ţ�
��ֻ���������̣���ѧ����
��ֻ�л�ѧ���̣�����������
�ۼ��л�ѧ����������������
FeSO4��7H2O�dz��õ����ۼ���������������ɺ��ɫ��״�����������ֳ�����________��
��3��ͨ�������̼��Ŀ����________��________��
��4������A��������______________________________________________________��
��5�����������У�________����д���и������ţ�������Ϊ����A�Ĵ���Ʒ��
��ClO2����Ũ��ˮ����SO2����Ũ����

��1��Դˮ�к�Ca2����Mg2����HCO3-��Cl���ȣ�����CaO������Ca��OH��2�������������ɸ����ֽⷴӦ����д����������һ����Ӧ�����ӷ���ʽ
_____________________________________________________________��
��2���������ۼ����Գ�ȥ���е���������������ù�����________����д���и������ţ�
��ֻ���������̣���ѧ����
��ֻ�л�ѧ���̣�����������
�ۼ��л�ѧ����������������
FeSO4��7H2O�dz��õ����ۼ���������������ɺ��ɫ��״�����������ֳ�����________��
��3��ͨ�������̼��Ŀ����________��________��
��4������A��������______________________________________________________��
��5�����������У�________����д���и������ţ�������Ϊ����A�Ĵ���Ʒ��
��ClO2����Ũ��ˮ����SO2����Ũ����
��1��HCO3-��OH��=CO32-��H2O
[��Ca2����HCO3-��OH��=CaCO3����H2O
��Ca2����2HCO3-��2OH��=CaCO3����CO32-��2H2O����Mg2����2OH��=Mg��OH��2��]
��2���ۡ�Fe��OH��3����3����ȥ�����ӡ�������Һ��ȡ���4��ɱ����������5����
[��Ca2����HCO3-��OH��=CaCO3����H2O
��Ca2����2HCO3-��2OH��=CaCO3����CO32-��2H2O����Mg2����2OH��=Mg��OH��2��]
��2���ۡ�Fe��OH��3����3����ȥ�����ӡ�������Һ��ȡ���4��ɱ����������5����
������֪��Դˮ�м���CaO�����ĸ��ֽⷴӦ�У�HCO3-��OH��=CO32-��H2O��Ca2����HCO3-��OH��=CaCO3����H2O��Mg2����2OH��=Mg��OH��2��������д��һ�����ɡ��������ۼ���ȥ���е���������������ù����Ǽ��н������������Ҳ�л�ѧ��Ӧ�����Լ��л�ѧ���������������̡�FeSO4��7H2O��������ɺ��ɫFe��OH��3��״������ͨ�������̼��Ŀ���dz�ȥ�����Ӳ�������Һ��ȡ�����AӦ����������������ɱ�����������Կ����Ҿ���ǿ�����Ե�ClO2���档

��ϰ��ϵ�д�
 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ