��Ŀ����
����Ŀ���������������ж�����Ӧ���ڹ��߱��ʷ��棬��NaClO3��CH3OH (�е�64.7��) �ڴ�����61��ʱ������Ӧ�õ�ClO2��ʵ��װ������ͼ��(��֪ClO2 ���ȶ��Խϲ���ȶ�������ClO2��ʹ��ʱ�����ͷų�ClO2)������������:
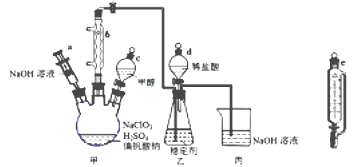
��1������b������Ϊ________����������_____________��
��2����Ӧ�м״�������Ϊ����(HCOOH)��д���Ʊ�ClO2�Ļ�ѧ����ʽ____________________��
��3����װ���в�ȡ�ļ��ȷ�ʽ��________________������μӼ״����ٶȹ��죬����ɵĺ��____________��
��4��ijͬѧ���齫����װ���еķ�Һ©��c��Ϊ��ѹ©��������Ϊ����������______________________��
��5��ʵ���������������a ע��һ������NaOH��Һ����һ��ʱ���ٲ�ж��������Ŀ����____________��
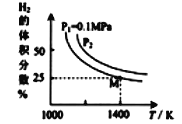
��6���±��������ȶ���������ͷ�ClO2��Ũ����ʱ��ı仯���ݣ�����������ӣ�ұ��ʣ�����ΪЧ���Ϻõ��ȶ�����_______( ��"1������2��)��ԭ����________________________________��
ʱ��/�� �ȶ��� | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 |
�ȶ���1 | 80 | 150 | 80 | 20 | 10 | 5 | 0 | 0 | 0 |
�ȶ���2 | 40 | 52 | 52 | 52 | 52 | 50 | 48 | 47 | 20 |
��7��ijͬѧ��ʵ���Ҳⶨij���ʼ���ClO2��������ʵ��������£���ά��ƿ�м���������KI��Һ���ټ���5mLϡ���ᣬȡ15ml���ʼ�����ƿ�С�ClO2��KI��Ӧ�����ӷ���ʽΪ:2ClO2+10I-+8H+==2C1-+5I2+4H2O����0.1000 mol/L Na2S2O3����Һ�ζ��ⵥ��(I2+2S2O32-=2I-+S4O62-) ���ﵽ�ζ��յ�ʱ��ȥ18.00mlNa2S2O3����Һ����øñ��ʼ���ClO2�ĺ���Ϊ________g��L-1
���𰸡� ���������� ���������״� 4NaClO3+CH3OH+2H2SO4![]() 2Na2SO4+4ClO2+HCOOH(H2CO2)+3H2O ˮԡ���� �ȶ�������������ClO2 ������Һ��˳����������ֹ�״��ӷ� ����Ӧֹͣ�������ն����ClO2 2 �ͷŵ�ClO2Ũ���ȶ�������ʱ�䳤 1.62
2Na2SO4+4ClO2+HCOOH(H2CO2)+3H2O ˮԡ���� �ȶ�������������ClO2 ������Һ��˳����������ֹ�״��ӷ� ����Ӧֹͣ�������ն����ClO2 2 �ͷŵ�ClO2Ũ���ȶ�������ʱ�䳤 1.62
���������������������ͼʾ��������b�����ƣ��״��е���ӷ���(2)�״�������Ϊ����(HCOOH)��NaClO3����ԭΪClO2�����ݵ�ʧ�����غ���ƽ����ʽ����3������NaClO3��CH3OH(�е�64.7��)�ڴ�����61��ʱ������Ӧ�������ȷ���������μӼ״����ٶȿ죬����ClO2�����ʿ�����4����ѹ©����ƽ����ѹ����5���������������ж�����ֹ��Ⱦ����6���ͷŵ�ClO2Ũ��Խ�ȶ���Ч��Խ������7�����ݹ�ϵʽClO2![]() 5Na2S2O3������
5Na2S2O3������
��������1������ͼʾ����b���������������������������������������������״���(2)�״�������Ϊ����(HCOOH)��NaClO3����ԭΪClO2����Ӧ����ʽ��4NaClO3+CH3OH+2H2SO4![]() 2Na2SO4+4ClO2+HCOOH+3H2O����3������NaClO3��CH3OH(�е�64.7��)�ڴ�����61��ʱ������Ӧ�����Լ��ȷ�ʽΪˮԡ����������μӼ״����ٶȿ죬����ClO2�����ʿ����ȶ�������������ClO2����4����ѹ©����ƽ����ѹ��������Һ��˳����������ֹ�״��ӷ�����5���������������ж���ע������������Һ�ܽ���Ӧֹͣ�������ն����ClO2����6���ͷŵ�ClO2Ũ���ȶ�������ʱ�䳤���ȶ���Ч���������ݱ���������Ч���Ϻõ��ȶ�����2����7���豣�ʼ���ClO2�ĺ�����xg��L-1
2Na2SO4+4ClO2+HCOOH+3H2O����3������NaClO3��CH3OH(�е�64.7��)�ڴ�����61��ʱ������Ӧ�����Լ��ȷ�ʽΪˮԡ����������μӼ״����ٶȿ죬����ClO2�����ʿ����ȶ�������������ClO2����4����ѹ©����ƽ����ѹ��������Һ��˳����������ֹ�״��ӷ�����5���������������ж���ע������������Һ�ܽ���Ӧֹͣ�������ն����ClO2����6���ͷŵ�ClO2Ũ���ȶ�������ʱ�䳤���ȶ���Ч���������ݱ���������Ч���Ϻõ��ȶ�����2����7���豣�ʼ���ClO2�ĺ�����xg��L-1
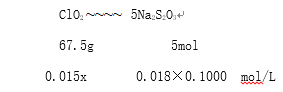
![]() ��x=1.62��
��x=1.62��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����ǹ�ҵ���Ʊ�Na2S2O3�ķ���֮һ����Ӧԭ��Ϊ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2���÷�Ӧ��H��0����ij�о�С����ʵ��������Ʊ�Na2S2O3��5H2O�������¡�

��1������װ����ͼ��ʾ��

��װ��B�������Ǽ���װ��A��SO2������Ч�ʣ�B���Լ���_____________������SO2����Ч�ʵ͵�ʵ��������B����Һ______________��
��Ϊ��ʹSO2������������ȫ���ڲ��ı�A����ҺŨ�ȡ�����������£����˼�ʱ���跴Ӧ���⣬���ɲ�ȡ�ĺ�����ʩ��______________������дһ����
��2�����豾ʵ�����õ�Na2CO3������NaCl��NaOH�����ʵ�鷽�����м��顣������ʱCaCO3������Һ��pH��10.2������ѡ�Լ���������ϡ���ᡢAgNO3��Һ��CaCl2��Һ����̪��Һ������ˮ��pH�ơ��ձ����Թܡ��ι�
��� | ʵ����� | Ԥ������ | ���� |
�� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ⣬____________ �� | �а�ɫ�������� | ��Ʒ��NaCl |
�� | ��ȡ������Ʒ���ձ��У�������������ˮ����ֽ����ܽ⣬_______�� | ���ɫ�������ɣ��ϲ���ҺpH>10.2 | ��Ʒ��NaOH |
��3��Na2S2O3��Һ�Ƕ���ʵ���еij����Լ����ⶨ��Ũ�ȵĹ������£�
��һ����ȷ��ȡa g KIO3(��Է���������214)���������Һ��
�ڶ������������KI�����H2SO4��Һ���μ�ָʾ����
����������Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��Һ�����Ϊv mL����c(Na2S2O3)��______mol��L-1����ֻ�г���ʽ���������㣩
��֪��IO3-+I-+6H+=3I2+3H2O 2S2O32-+I2=S4O62-+2I-����ͬѧʢװNa2S2O3��Һ֮ǰδ��ϴ��������õ�Na2S2O3��Ũ�ȿ���________��������Ӱ��������ƫ��������ƫ����)����ͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�Na2S2O3��Ũ�ȿ���________(������Ӱ�������� ƫ��������ƫ����)��