��Ŀ����
��10�֣��״���һ�ַdz��õ�ȼ�ϡ��۵�-97.8��C���е�64.5��C��һ�������£�
CO��H2��Ӧ���Ƶü״���CO+2H2  CH3OH
CH3OH
ͼ1��ʾ�÷�Ӧ���й����е������仯��
ͼ2��ʾ100��C�������Ϊ2L�ĺ��������м���4molH2��һ������CO��CO��CH3OH��g����Ũ����ʱ��仯�����
��1����֪CO��ȼ����Ϊ283kJ/mol��H2��ȼ����Ϊ285.8kJ/mol�����ͼ1д��Һ̬CH3OHȼ���ȵ��Ȼ�ѧ����ʽ ��
��2��������ͼ2���㣺���¶��£���ӦCO��g��+2H��g�� CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ���� �����ı����������������COת���ʵ��� ��
CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ���� �����ı����������������COת���ʵ��� ��
A�������¶� B���Ӵ���
C�����������ʹ��ϵѹǿ���� D���ٳ���1molCO��2molH2
E�����º��ݸ�Ϊ���º�ѹ
��3����֪��CH3OH������һ��������ת��ΪHCOOH��HCOOH��CH3COOH�������ơ�25��C��0.1mol/LHCOOH��Һ��pH>1�������£���0.1mol/L��HCOOH��Һ�еμ�NaOH��Һ������Һ������Ũ�ȹ�ϵ���㣺c��HCOO-��<c��Na+��ʱ����Ӧ���������Ϊ ��������ĸ��
A��NaOH���㣬HCOOHʣ��
B��HCOOH��NaOHǡ����ȫ��Ӧ
C��NaOH����
��1��CH3OH(l) + 3/2O2(g) = CO2(g) + 2H2O (l) ��H= -725.6kJ/mol
��2��12 0.5 A��D��E ��3��A��B��C
����

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� CH3OH
CH3OH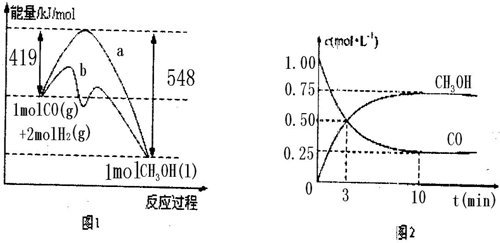
 CH3OH��g����H��0��ƽ�ⳣ��Ϊ
CH3OH��g����H��0��ƽ�ⳣ��Ϊ CH3OH
CH3OH
 CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ����
�����ı����������������COת���ʵ���
��
CH3OH��g����ƽ�ⳣ��Ϊ ��10min�������ڵ�ѹǿ��Ϊԭ����
�����ı����������������COת���ʵ���
��