��Ŀ����
��16�֣������Ѱ�ҵ�ĸ���ƷFeSO4����TiO2+��Al3+������������ؼ��ߴ���ϸ�����������乤���������£�
��1������FeSO4�Ƿ����в��������ķ����� ��
��2����֪����1�õ�����������Ҫ�ɷ���Al(OH)3��H2TiO3���벹�仯ѧ����ʽ��
TiOSO4 + ��H2SO4 + H2TiO3�������۵������У��ٳ�ȥ��Һ�е�Fe3+���� ��
��3��������Ӧ�����ӷ���ʽ�� ��
��4���������̵ķ�Ӧ�¶�Ϊ40�棬�¶Ȳ��˹��ߵ�ԭ����˿��Ƴ����������⣬���� ��FeC2O4���ɺ�Ϊ��߲�Ʒ���ȣ����������ҺpH��2����pH���ͣ�����FeC2O4�IJ���______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��5������2�õ�����Һ������Ũ���� ��ϴ�ӿɵõ�����Ʒ�������ʿ����� (д��һ����;)��
(16�֣�ÿ��2�֣���1��ȡ��������Ʒ���Թ��У�����������ˮ�ܽ⣬�μ�1��2��KSCN��Һ������Һ�Ժ�ɫ���������в���������������ȫ��1�֣�
��2��2H2O����д��H2O ��1�֣� ����Һ�е�H+��Ӧ��ʹAl3+ˮ����ȫ����Al(OH)3����
��3��2NH3?H2O + Fe2+ ��Fe(OH)2��+2NH4+����ƽ�����©�������ſ�1�֣�
��4��NH3?H2O��Fe(OH)2���ȶ��ֽ⣻ƫ�� ��5����ȴ�ᾧ�����ˣ�����
���������������1���������ӱ��������������ӣ�����ͨ��������������֤�Ƿ����������Լ���FeSO4�Ƿ����в��������ķ�����ȡ��������Ʒ���Թ��У�����������ˮ�ܽ⣬�μ�1��2��KSCN��Һ������Һ�Ժ�ɫ���������в���������
��2�����ݷ�Ӧǰ��ԭ���غ��֪����ȱ��2����ˮ��������Һ�����ԣ��������к����������������Ҫ�õ������������뽵����Һ�����ԣ�����������һ����������Һ�е�H+��Ӧ��ʹAl3+ˮ����ȫ����Al(OH)3������
��3����Һ�е����������백ˮ��������������������������Ӧ�����ӷ���ʽΪ2NH3?H2O + Fe2+ ��Fe(OH)2��+2NH4+��
��4�����¶ȹ�����NH3?H2O��Fe(OH)2���ȶ��ֽ⣻��pH���ͣ�����FeC2O4�����ܽ⣬�Ӷ����²���ƫ�ͣ�
��5������2�õ�����Һ������Ũ������ȴ�ᾧ��ϴ�ӿɵõ�����Ʒ����泥������ʿ��������ʡ�
���㣺���������Ӽ��顢�����Ʊ�ʵ�鷽����Ƶ�

 �Ķ��쳵ϵ�д�
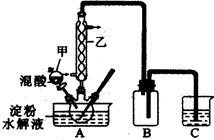
�Ķ��쳵ϵ�д���9�֣�����ֲ��纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ����������£�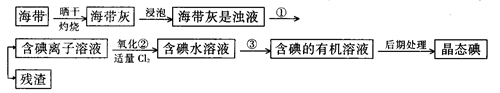
��1��ָ����ȡ��Ĺ������йص�ʵ��������ƣ�
�� ����______________________��
д��ʵ������йط�Ӧ�����ӷ���ʽ _______________________ ��
��2����ȡ��Ĺ����У��ɹ�ѡ����й��Լ���___________��
| A���ױ����ƾ� | B�����Ȼ�̼���� |
| C�����ͣ����� | D�����ͣ����� |
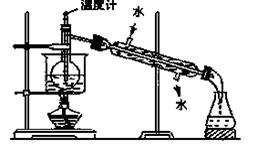
��3���Ӻ�����л���Һ����ȡ��ͻ����л��ܼ����辭������ָ����ͼʵ��װ���еĴ���֮������ �� �� ��
��4�����������������ʱ��ʹ��ˮԡ��ԭ����___________�����̬����___________��ۼ���
��14�֣�ijѧ��Ϊ�ⶨij�ռ���Ʒ��NaOH��������������������ʵ�飨��֪����Ʒ�к�������Na2CO3���ʣ�
a����250mL������ƿ�ж��ݣ����Ƴ�250mL�ռ���Һ��
b���ü�ʽ�ζ�����ȡ25.00mL�ռ���Һ����ƿ�У���������BaCl2��ҺʹNa2CO3��ȫת���BaCO3����뼸�η�ָ̪ʾ����
c������ƽ��ȷ��ȡ�ռ���Ʒ10.5g�����ձ���������ˮ�ܽ⣻
d�������ʵ���Ũ��Ϊ1.000mol/L�ı�������Һװ����ʽ�ζ����У�����Һ�棬���¿�ʼʱ�Ķ���Ȼ��ʼ�ζ���
e������ƿ�µ�һ�Ű�ֽ���ζ�����Һǡ�ñ�Ϊ��ɫΪֹ�����¶���������գ�
��1����ȷ���������˳���ǣ�����ĸ��գ�_____��_____��_____��_____��_______.
��2����ʽ����ʽ���ζ�����ʹ��ǰ����еĵ�һ��������____________����ѧ��ѧʵ�鳣��������ʹ��ǰ�͵ζ���ʹ������ͬ�����IJ�ͬ�ಣ���������� ��_______��
��3���ظ������ζ���������¼�������£�
| ʵ���� | ����Һ��H2SO4��(aq) Ũ�ȣ�mol/L�� | �ζ����ʱ���� ���V��mL�� | ������Һ��NaOH��(aq) ���V��mL�� |
| 1 | 1.000 | 11.00 | 25.00 |
| 2 | 1.000 | 12.04 | 25.00 |
| 3 | 1.000 | 12.18 | 25.00 |
������ʵ���У����в���������������ȷ������ɽ��ƫ�͵���__________________��
A��a��������δ����Һ��ȴ�����¾�ת�Ƶ�����ƿ�ж��ݡ�
B��c�������У�����ҩƷʱ������������̣�NaOH�������̡�
C���ζ��յ����ʱ���Ӷ�����
D����ʽ�ζ���ʹ��ǰ��δ�ñ�H2SO4��Һ��ϴ��
E����ƿˮϴ��δ�����ֱ��ʢ������Һ��
����ͬѧ�����ԭʵ�鷽���еķ�ָ̪ʾ����Ϊ����ָʾ��������Ϊ�Ƿ���У�________������С������С�����������ü���Ϊָʾ������ⶨ�����Σ�________���ƫ�ߡ� ��ƫ�͡�����ȷ����
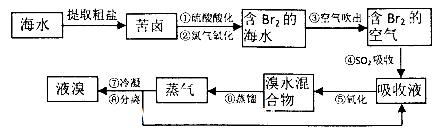
��15�֣���ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ�����Ҫ�ɷ�ΪCuFeS2������һ����Ȼ��ͭ������SiO2����Ϊ�˲ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺
�ֳ�ȡ��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ��������½������գ�����Cu��Fe3O4��SO2���壬ʵ���ȡd����Һ�� ������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00ml����ش��������⣺
������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00ml����ش��������⣺
��1��������Ʒ���õ�����Ϊ_____(�������ƽ��������ƽ��)������Ʒ��ϸ���ٷ�Ӧ����Ŀ����_______ ��
��2��װ��a��c�����÷ֱ���____��____�����ţ���
| A����ȥSO2���� | B����ȥ�����е�ˮ���� | C�������������� |
| D�������ڹ۲�������� E.��ȥ��Ӧ���������� |
��4��ͨ�������֪���û�ͭ��Ĵ���Ϊ________��
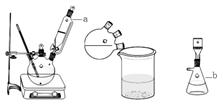
��5��������ͼװ���������ʵ��װ��d��ͬ�����Դﵽʵ��Ŀ�ĵ���____������ţ���

��6������ԭװ��d�е���Һ��ΪBa(OH)2����õĻ�ͭ�����Ϊ��1%������ʵ���������ȷ�����ܵ�ԭ����Ҫ��_____________________________________________��



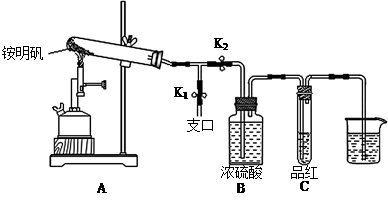
 3H2C2O4��9NO2����3NO����9H2O
3H2C2O4��9NO2����3NO����9H2O