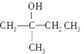
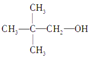
��Ŀ����
����Ŀ��(1)������䳣��Һ̬��(N2H4)Ϊȼ�ϣ�Һ̬��������Ϊ��ȼ������֪��16gҺ̬����Һ̬��������ǡ����ȫ��Ӧ���ɵ�����Һ̬ˮʱ�ų�321kJ��������д��Һ̬N2H4��Һ̬H2O2��Ӧ���Ȼ�ѧ����ʽ__________��
(2)��֪��N2H4(l)��O2(g)=N2(g)��2H2O(g)����(N2H4)������ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20%~30%��KOH��Һ���õ�طŵ�ʱ�������ĵ缫��Ӧʽ��_______��
(3)��Ũ�Ⱦ�Ϊ0.10 mol��L-1��CH3COOH��Һ��NaOH��Һ�������ϣ����û����Һ�и�������Ũ�ȴ�С˳��_______________��
(4)��֪ij�¶��£���ˮ�е�c(H+)= 2.0��10-7 mol/L�����¶��£��ⶨij������Һ��c(SO42-)=5��10-6mol/L����������Һ����ˮ�������OH-Ũ��Ϊ_________________��
(5)��25 ��ʱ����V mL pH��m��HNO3�еμ�pH��n��KOH��Һ10V mLʱ����Һ��n(NO3-)=10 n(K��)����m��n��ֵΪ____________��
���𰸡�N2H4(l)��2H2O2(l)=N2(g)��4H2O(l) ��H����642 kJ��mol-1 N2H4��4e����4OH����N2��4H2O c(Na+)��c(CH3COO��)��c(OH��)��c(H+) 4��109 mol��L1 12
��������
(1)����16gҺ̬����Һ̬��������ǡ����ȫ��Ӧ���ɵ�����Һ̬ˮʱ�ų�321kJ����������1molҺ̬��32g�ų�642kJ������������д�ȷ�Ӧ����ʽ��
(2)�����ó�N2H4���ϼ���������������Ӧ���ɵ�����
(3)������Ӧ������ʼ���Һ����ԣ��ٷ�������Ũ�ȴ�С˳��
(4)������¶���Kw���ٵõ�ij������Һ��c(H+)���ٸ��ݹ�ʽ����Һ�е�OH��Ũ�ȼ�Ϊˮ�������OH��Ũ�ȡ�
(5)�ȱ�ʾ��HNO3��c(H+)��pH��n��KOH��Һc(OH��)��������Һ��n(NO3��)=10 n(K��)�м���ʽ�ó�m��n ��ֵ��
(1)16gҺ̬����Һ̬��������ǡ����ȫ��Ӧ���ɵ�����Һ̬ˮʱ�ų�321kJ������N2H4��2H2O2 = N2 ��4H2O��1molҺ̬��32g�ų�642kJ�����������Һ̬N2H4��Һ̬H2O2��Ӧ���Ȼ�ѧ����ʽN2H4(l)��2H2O2(l)=N2(g)��4H2O(l) ��H����642 kJ��mol1���ʴ�Ϊ��N2H4(l)��2H2O2(l)=N2(g)��4H2O(l) ��H����642 kJ��mol1��
(2)��(N2H4)������ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20%~30%��KOH��Һ�����ݷ�Ӧ����ʽN2H4(l)��O2(g)=N2(g)��2H2O(g)��N2H4���ϼ��������������ŵ�ʱ�������ĵ缫��Ӧʽ��N2H4��4e����4OH����N2��4H2O���ʴ�Ϊ��N2H4��4e����4OH����N2��4H2O��
(3)��Ũ�Ⱦ�Ϊ0.10 mol��L1��CH3COOH��Һ��NaOH��Һ�������ϣ�����ΪCH3COONa����Һ�Լ��ԣ���Ҫ�Ǵ����ˮ�⣬������û����Һ�и�������Ũ�ȴ�С˳��c(Na+)��c(CH3COO��)��c(OH��)��c(H+)���ʴ�Ϊ��c(Na+)��c(CH3COO��)��c(OH��)��c(H+)��
(4)��֪ij�¶��£���ˮ�е�c(H+)= 2.0��107 mol��L1��Kw = c(H+)c(OH��) = 2.0��107�� 2.0��107 =4��1014�����¶��£��ⶨij������Һ��c(SO42)=5��106 mol��L1��c(H+)=1��105 mol��L1�����������Һ�е�OH��Ũ��![]() ��ˮ�������OH��Ũ��4��109 mol��L1���ʴ�Ϊ��4��109 mol��L1��
��ˮ�������OH��Ũ��4��109 mol��L1���ʴ�Ϊ��4��109 mol��L1��
(5)��25 ��ʱ����V mL pH��m��HNO3��c(H+)= 1��10m mol��L1���μ�pH��n��KOH��Һ[��c(OH��) = 1��10(n14) mol��L1]10V mLʱ����Һ��n(NO3��)=10 n(K��)�����1��10m mol��L1��V��103L = 10��1��10(n14) mol��L1��10V��103L���õ�m��n =12���ʴ�Ϊ��12��

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�