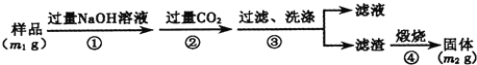
��Ŀ����
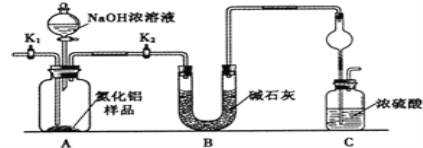
����Ŀ��ijʵ��С����MgBr2ϡ��Һģ��Ӻ�ˮ����ȡ��ˮ�Ȼ�þ���嵥�ʣ�ʵ���������£�����˵������ȷ����
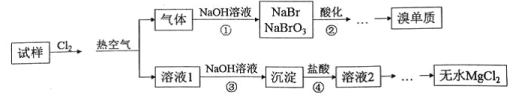
A. ������е�NaOH��Һ������Na2CO3��Na2SO3��Һ����
B. ����ڿ��������ữ�����������ӷ�ӦΪ5Br��+BrO3��+6H+=3Br2+3H2O
C. ����۰������ˡ�ϴ�ӵȲ���������AgNO3��Һ�����Ƿ�ϴ�Ӹɾ�
D. ������ۢܣ���Һ1����Һ2����Ҫ�ɷ�����ͬ�ģ���Ũ�����нϴ����
���𰸡�A
��������
A�������м���Cl2������2Br����Cl2=2Cl=��Br2�������ȿ���������������Ϊ��������Br2��Na2CO3����3Br2��3Na2CO3=5NaBr��NaBrO3��3CO2�����������绯��Ӧ��Na2SO3���л�ԭ�ԣ�����Br2����������Ӧ���õ�NaBr��Na2SO4�����������⣬NaOH������Na2SO3���棬��A����B������ڿ��������ữ������5Br����BrO3����6H��=3Br2��3H2O����B��ȷ��C����Һ1��������Ҫ�ɷ���MgCl2������NaOH��Һ������Mg2����2OH��=Mg(OH)2����Ȼ����ˡ�ϴ�ӡ�����Ȳ�����Mg(OH)2���溬������Cl������˼����Ƿ�ϴ�Ӹɾ���AgNO3��Һ����C��ȷ��D������ۢ��൱�ڸ�������D��ȷ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�