��Ŀ����
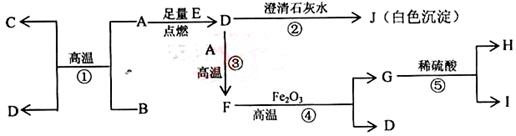
��15�֣���A��B��C��D��E��F��G����Ԫ�أ������������������ƶϣ�
��A��B��C��ͬһ���ڵĽ���Ԫ�أ���֪ԭ�Ӻ������3�����Ӳ㣬A��ԭ�Ӱ뾶�����������������ԭ�Ӱ뾶B��C��
��D��E��G�Ƿǽ���Ԫ�أ����Ƕ����Ը��⻯��������̬�⻯��HD��HE��HG��������ʱ��G�ĵ���������������Ͼͱ�ը��D�ĵ�����Һ�壬E�ĵ����ǹ��壻
��F�ĵ����ڳ����������壬���ʺ��ȶ����dz�������������塣
��1��A�������� ��Bλ�����ڱ��е� ���� �壬C��ԭ�ӽṹʾ��ͼ��
��ÿ��l�֣���
��2��E�ĵ�����ɫ�� ��1�֣���
��3��AԪ����DԪ���γɻ�����ĵ���ʽ�� ��2�֣���
��4��G�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ�� ��2�֣���
��5��F��Ԫ�ط����� ��1�֣���
��6������������Ԫ���У�����������Ӧ��ˮ���������ǿ�Ļ�ѧʽ�� ��������ǿ�Ļ�ѧʽ�� ����̬�⻯�����ȶ��Ļ�ѧʽ�� ��ÿ��l�֣���
��7����C���������Ӧ��ˮ����Ͷ�뵽A���������Ӧ��ˮ�����з�Ӧ�����ӷ���ʽ��
��2�֣���
��A��B��C��ͬһ���ڵĽ���Ԫ�أ���֪ԭ�Ӻ������3�����Ӳ㣬A��ԭ�Ӱ뾶�����������������ԭ�Ӱ뾶B��C��
��D��E��G�Ƿǽ���Ԫ�أ����Ƕ����Ը��⻯��������̬�⻯��HD��HE��HG��������ʱ��G�ĵ���������������Ͼͱ�ը��D�ĵ�����Һ�壬E�ĵ����ǹ��壻
��F�ĵ����ڳ����������壬���ʺ��ȶ����dz�������������塣
��1��A�������� ��Bλ�����ڱ��е� ���� �壬C��ԭ�ӽṹʾ��ͼ��
��ÿ��l�֣���
��2��E�ĵ�����ɫ�� ��1�֣���
��3��AԪ����DԪ���γɻ�����ĵ���ʽ�� ��2�֣���
��4��G�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ�� ��2�֣���
��5��F��Ԫ�ط����� ��1�֣���
��6������������Ԫ���У�����������Ӧ��ˮ���������ǿ�Ļ�ѧʽ�� ��������ǿ�Ļ�ѧʽ�� ����̬�⻯�����ȶ��Ļ�ѧʽ�� ��ÿ��l�֣���
��7����C���������Ӧ��ˮ����Ͷ�뵽A���������Ӧ��ˮ�����з�Ӧ�����ӷ���ʽ��
��2�֣���
��1���� 3 ��
 A
A 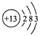
��2���Ϻ�ɫ
��3��
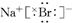
��4��2F2+2H2O
 4HF+O2
4HF+O2��5��He
��6��NaOH HBrO4 HF
��7��Al��OH��3+OH��

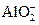 +2H2O
+2H2O
������Ԫ���ƶ����Ǹ߿������Ŀ��㣬���ʱһ��Ҫ��ϵԪ�����ڱ������Ԫ�������ڱ��е�λ�ã�Ȼ������Ԫ�ء�λ�������ԡ��Ĺ�ϵ�����ۺ��ƶϡ����Ҫ����Ҫ��Ϥͬ���ڡ�ͬ����Ԫ�����ʵĵݱ���ɡ�

��ϰ��ϵ�д�
�����Ŀ

 ��CԪ�صĵ�һ��������ͬ��Ԫ��������Ҹ���ͬ�����������ڵ�Ԫ�أ���
��CԪ�صĵ�һ��������ͬ��Ԫ��������Ҹ���ͬ�����������ڵ�Ԫ�أ���