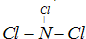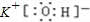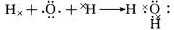��Ŀ����
����A��B��C��D��E��X��Y��M�����ʣ����Ǿ�Ϊ�������һ�������¿ɷ�������ת����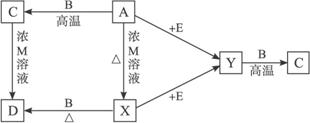
���У�X��YΪ����˫ԭ�����嵥��(XΪ��ɫ����)��BΪ�����������ʣ�������C����ǿ���ǿ�EΪ�����ֳ���Ԫ��(��ԭ�Ӹ���1��1)��ɵ�Һ�壻AΪ��ɫ�������������ת����ֻд������һ�����������������û��д��(Ҳ�п��ܷ�Ӧֻ��һ��������)��
�Իش�
(1)д����ѧʽ��X____________��E______________��
(2)д�����ӷ���ʽ��
A��X��_____________________��C��D��__________________��
(3)X+E��Ӧ�Ļ�ѧ����ʽΪ________________________��
(1)Cl2 H2O2
(2)MnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O
Mn2++Cl2��+2H2O
Al2O3+6H+====2Al3++3H2O
(3)Cl2+H2O2====2HCl+O2
���������������֪XΪCl2����AΪMnO2��CΪ����������֪BΪ���������Ƴ�Y����������E��ΪH2O2��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
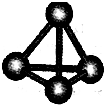 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��