��Ŀ����
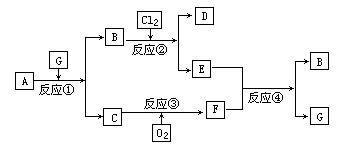
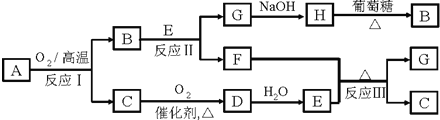
����A����ͼ��ʾת����ϵ��������Ϊ�������ʣ������£�����G��Ũ��Һ�з����ۻ���F����Һ��ֻ����һ�����ʣ��еķ�Ӧ������ˮ��Һ�н��У��еķ�Ӧ��������δȫ����������Ӧ����Ҳδע������
�������������������ע����������¸���ĸ���������ʿ��ܲ�ͬ����
(1)��һ�������AΪ���壻�������������ֱ�պȡA��G��Ũ��Һ��ʹ���ǽӽ�ʱ���д����������ɣ���Ϊ��ɫ��Ӧ�ʻ�ɫ�Ľ������ʣ�D��F����Һ���ʼ��ԡ���
��D���ҷ�Ӧ�����ӷ���ʽΪ___________________��
�������ӷ���ʽ��ʾF��Һ�Լ��Ե�ԭ��_______________________��
(2)�ڶ��������A�ǹ�ҵ�ϳ����Ŀ�ʯ���������ֵ���ֱ�ӻ��ϵõ���D��ˮ��Һ����HNO3�ữ��AgNO3��Һ�а�ɫ�������ɡ���
�ٹ�ҵ�ϣ���ӦI��__________�����豸���ƣ��н��У�
���õ���ʽ��ʾ��������γɹ��̣�_____________________��
��D���ҷ�Ӧ�����ӷ���ʽΪ__________________��
�ܷ�Ӧ��Ļ�ѧ����ʽΪ__________________��
(1)��һ�������AΪ���壻�������������ֱ�պȡA��G��Ũ��Һ��ʹ���ǽӽ�ʱ���д����������ɣ���Ϊ��ɫ��Ӧ�ʻ�ɫ�Ľ������ʣ�D��F����Һ���ʼ��ԡ���
��D���ҷ�Ӧ�����ӷ���ʽΪ___________________��
�������ӷ���ʽ��ʾF��Һ�Լ��Ե�ԭ��_______________________��
(2)�ڶ��������A�ǹ�ҵ�ϳ����Ŀ�ʯ���������ֵ���ֱ�ӻ��ϵõ���D��ˮ��Һ����HNO3�ữ��AgNO3��Һ�а�ɫ�������ɡ���
�ٹ�ҵ�ϣ���ӦI��__________�����豸���ƣ��н��У�
���õ���ʽ��ʾ��������γɹ��̣�_____________________��
��D���ҷ�Ӧ�����ӷ���ʽΪ__________________��
�ܷ�Ӧ��Ļ�ѧ����ʽΪ__________________��
(1)��2Al+2OH-+2H2O=2AlO2-+3H2������AlO2-+2H2O Al(OH)3+OH-
Al(OH)3+OH-
(2)�ٷ���¯����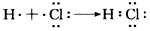 ����2Fe3++Fe=3Fe2+�� ��2SO2+O2
����2Fe3++Fe=3Fe2+�� ��2SO2+O2 2SO3
2SO3
 Al(OH)3+OH-
Al(OH)3+OH-(2)�ٷ���¯����
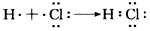 ����2Fe3++Fe=3Fe2+�� ��2SO2+O2
����2Fe3++Fe=3Fe2+�� ��2SO2+O2 2SO3
2SO3 
��ϰ��ϵ�д�
�����Ŀ
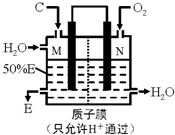
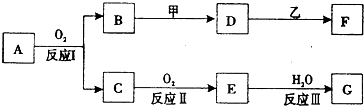
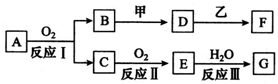
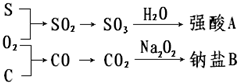 �������ʼ�����ͼ��ʾת����ϵ���ش��������⣺
�������ʼ�����ͼ��ʾת����ϵ���ش��������⣺