��Ŀ����
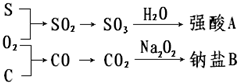 �������ʼ�����ͼ��ʾת����ϵ���ش��������⣺
�������ʼ�����ͼ��ʾת����ϵ���ش��������⣺��1��д��ǿ��A�ķ���ʽ
��2������B��ˮ��Һ�Լ��ԣ�˵����ԭ������ӷ���Ϊ��
��3��д��CO2��Na2O2��Ӧ��������B�Ļ�ѧ����ʽ��
��4�����1mol̼��ȫȼ�պ�ɷų�393.5KJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ��
��5������������
��6��д������CO2��SO2��һ�ַ�����
��������1������������ˮ��Ӧ�������
��2��̼����Ϊǿ��������ˮ���Լ��ԣ�
��3��CO2��Na2O2��Ӧ����̼���ƺ�������
��4�������Ȼ�ѧ����ʽ����д�������н��ע���ע���ʾۼ�״̬�Ͷ�Ӧ�ʱ䣻
��5��SO2������ˮ���������� H2SO4���γ����ꣻ
��6��SO2����Ư���ԣ�CO2û��Ư���ԣ�
��2��̼����Ϊǿ��������ˮ���Լ��ԣ�
��3��CO2��Na2O2��Ӧ����̼���ƺ�������
��4�������Ȼ�ѧ����ʽ����д�������н��ע���ע���ʾۼ�״̬�Ͷ�Ӧ�ʱ䣻
��5��SO2������ˮ���������� H2SO4���γ����ꣻ
��6��SO2����Ư���ԣ�CO2û��Ư���ԣ�
����⣺��1������������ˮ��Ӧ�������ᣬ����ǿ��AΪH2SO4���ʴ�Ϊ��H2SO4��
��2��̼����Ϊǿ��������ˮ���Լ��ԣ���ˮ�����ӷ���Ϊ��CO32-+H2O?HCO3-+OH-���ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��
��3��CO2��Na2O2��Ӧ����̼���ƺ��������䷴Ӧ�Ļ�ѧ����ʽΪ2CO2+2Na2O2=2Na2CO3+O2�����ʴ�Ϊ��2CO2+2Na2O2=2Na2CO3+O2����
��4��1mol̼��ȫȼ�պ�ɷų�393.5KJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ��C��s��+O2��g��=CO2��g����H=-393.5kJ/mol��
�ʴ�Ϊ��C��s��+O2��g��=CO2��g����H=-393.5kJ/mol��
��5��SO2������ˮ���������� H2SO4���γ����꣬����SO2�������γɵ���Ҫԭ�ʴ�Ϊ��SO2��
��6��SO2����Ư���ԣ�CO2û��Ư���ԣ���˽���������ֱ�ͨ��Ʒ����Һ����ʹƷ����Һ��ɫ����SO2���ʴ�Ϊ����ʹƷ����Һ��ɫ����SO2��
��2��̼����Ϊǿ��������ˮ���Լ��ԣ���ˮ�����ӷ���Ϊ��CO32-+H2O?HCO3-+OH-���ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��
��3��CO2��Na2O2��Ӧ����̼���ƺ��������䷴Ӧ�Ļ�ѧ����ʽΪ2CO2+2Na2O2=2Na2CO3+O2�����ʴ�Ϊ��2CO2+2Na2O2=2Na2CO3+O2����
��4��1mol̼��ȫȼ�պ�ɷų�393.5KJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ��C��s��+O2��g��=CO2��g����H=-393.5kJ/mol��
�ʴ�Ϊ��C��s��+O2��g��=CO2��g����H=-393.5kJ/mol��
��5��SO2������ˮ���������� H2SO4���γ����꣬����SO2�������γɵ���Ҫԭ�ʴ�Ϊ��SO2��
��6��SO2����Ư���ԣ�CO2û��Ư���ԣ���˽���������ֱ�ͨ��Ʒ����Һ����ʹƷ����Һ��ɫ����SO2���ʴ�Ϊ����ʹƷ����Һ��ɫ����SO2��
���������⿼����C��S����Ԫ�صĻ����� ���ʣ��漰���ʵ�ת����ˮ��ԭ����Ӧ�á�����ʽ����д���Ȼ�ѧ��ʽ�����ʵļ���ȣ��������ݽ϶࣬���ػ���֪ʶ�Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ
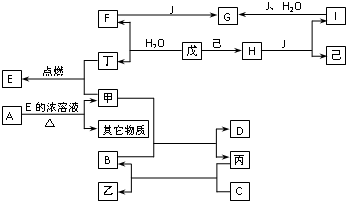 ��֪A��B��C��D��E��F��G��H��I��J�ǻ�����ס��ѡ����������졢���ǵ��ʣ����У�F��H��G��I��ɫ��Ӧ����ʻ�ɫ��B��C��D��ɫ��Ӧ���ܲ����۲�������ɫ��H�ǵ���ɫ���壮�����ʼ�����ͼ��ʾ��ת����ϵ��
��֪A��B��C��D��E��F��G��H��I��J�ǻ�����ס��ѡ����������졢���ǵ��ʣ����У�F��H��G��I��ɫ��Ӧ����ʻ�ɫ��B��C��D��ɫ��Ӧ���ܲ����۲�������ɫ��H�ǵ���ɫ���壮�����ʼ�����ͼ��ʾ��ת����ϵ��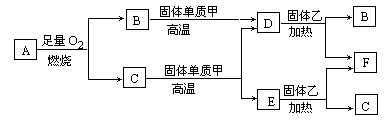
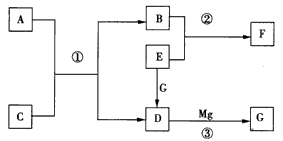
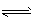 B(s)+D(g) ��H=akJ/mol(a>0)�����¶���ƽ�ⳣ��K=0.263��������1 mol B�������յ�����____��ѡ����ڡ��������ڡ���С�ڡ���a kJ�������������A����C��ת����__ ��ѡ����ߡ��������䡱���͡�������������ѹǿ������ʱ��仯����÷�Ӧ__ ��ѡ��ﵽ������δ�ﵽ����һ���ﵽ������ѧƽ��״̬���÷�Ӧ�ﵽ��ѧƽ��״̬ʱ����c( C) =0. 100 mol/L����c(D)=___mol/L��
B(s)+D(g) ��H=akJ/mol(a>0)�����¶���ƽ�ⳣ��K=0.263��������1 mol B�������յ�����____��ѡ����ڡ��������ڡ���С�ڡ���a kJ�������������A����C��ת����__ ��ѡ����ߡ��������䡱���͡�������������ѹǿ������ʱ��仯����÷�Ӧ__ ��ѡ��ﵽ������δ�ﵽ����һ���ﵽ������ѧƽ��״̬���÷�Ӧ�ﵽ��ѧƽ��״̬ʱ����c( C) =0. 100 mol/L����c(D)=___mol/L��