ЬтФПФкШн
ЁОЬтФПЁПЛЏбЇЗДгІЖМгаФмСПБфЛЏЁЃБћЭщ(C3H8)ЪЧвЛжжгХСМЕФШМСЯЃЌШчЭМЪЧвЛЖЈСПБћЭщдкГЃЮТГЃбЙЯТЭъШЋШМЩеЩњГЩCO2КЭ1 mol H2O(l)Й§ГЬжаЕФФмСПБфЛЏЭМЁЃЪдЛиД№ЯТСаЮЪЬтЃК
(1)аДГіБћЭщШМЩеШШЕФШШЛЏбЇЗНГЬЪНЃК___ЁЃ
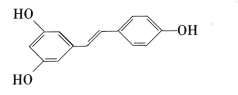
(2)ЖўМзУб(CH3OCH3)ЪЧвЛжжаТаЭШМСЯЃЌгІгУЧАОАЙуРЋЁЃ1 molЖўМзУбЭъШЋШМЩеЩњГЩCO2КЭвКЬЌЫЎЗХГі1455 kJЕФШШСПЁЃШє1 molБћЭщКЭЖўМзУбЕФЛьКЯЦјЬхЭъШЋШМЩеЩњГЩCOКЭвКЬЌЫЎЙВЗХГі1607 kJЕФШШСПЃЌдђЛьКЯЦјЬхжаБћЭщКЭЖўМзУбЕФЮяжЪЕФСПжЎБШЮЊ___ЁЃ
ЁОД№АИЁПC3H8(g)+5O2(g)=3CO2(g)+4H2O(l) ЁїH= -2215.0 kJ/mol 1ЃК4
ЁОНтЮіЁП
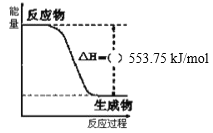
(1)БћЭщШМЩеЗДгІЪЧЗХШШЕФЃЌЫљвдЁїH<0ЃЌЗжЮіЭМЯѓЕУЕНЩњГЩ1 molЫЎЕФьЪБфЁїH=-553.75 kJ/molЃЌвРОнШШЛЏбЇЗНГЬЪНЪщаДЗНЗЈаДГіЃЌзЂвтЮяжЪОлМЏзДЬЌЃЌЖдгІСПЯТЕФьЪБфЃЛ
(2)вРОнШШЛЏбЇЗНГЬЪННсКЯЛьКЯЦјЬхЮяжЪЕФСПКЭЗХШШСаЪНМЦЫуЕУЕНЖўМзУбКЭБћЭщЮяжЪЕФСПжЎБШЁЃ
(1)ЭМЯѓЪЧвЛЖЈСПБћЭщЭъШЋШМЩеЩњГЩCO2КЭ1 mol H2O(l)Й§ГЬжаЕФФмСПБфЛЏЭМЃЌБћЭщШМЩеЗДгІЪЧЗХШШЕФЃЌЫљвдЁїH=-553.75 kJ/molЃЛБћЭщЭъШЋШМЩеЩњГЩCO2КЭ1 mol H2O(l)ЗДгІЗХШШЁїH= -553.75 kJ/molЃЌгЩгкБћЭщЛЏбЇЪНЪЧC3H8ЃЌдђ1 molБћЭщЭъШЋШМЩеВњЩњ4 mol H2OЃЌЗДгІЗХГіШШСПQ=4 molЁС553.75 kJ/mol=2215.0 kJ/molЃЌЙЪИУЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊЃКC3H8(g)+5O2(g)=3CO2(g)+4H2O(l) ЁїH=-2215.0 kJ/molЃЛ
(2)1 molЖўМзУбЭъШЋШМЩеЩњГЩCO2КЭвКЬЌЫЎЗХГі1455 kJШШСПЃЌ1 molБћЭщЭъШЋШМЩеЗХГі2215.0 kJЕФШШСПЃЌШє1 molБћЭщКЭЖўМзУбЕФЛьКЯЦјЬхЭъШЋШМЩеЩњГЩCO2КЭвКЬЌЫЎЙВЗХГі1607 kJШШСПЃЌЩш1 molЛьКЯЦјЬхжаЖўМзУбЮяжЪЕФСПxЃЌБћЭщЮяжЪЕФСПЮЊ(1-x)ЃЌ1455x kJ+2215.0(1-x) kJ=1607 kJЃЌНтЕУx=0.8ЃЌдђЛьКЯЦјЬхжаБћЭщЮяжЪЕФСПЮЊ0.2 molЃЌЙЪЛьКЯЦјЬхжаБћЭщКЭЖўМзУбЮяжЪЕФСПжЎБШn(C3H8)ЃКn(CH3OCH3)=0.2 molЃК0.8 mol=1ЃК4ЁЃ

ЁОЬтФПЁПзАжУaЁЂbЁЂcжаЗжБ№ЪЂгаЪдМС1ЁЂ2ЁЂ3ЃЌгУШчЭМЫљЪОЕФзАжУНјааЪЕбщ(МаГжвЧЦїТдШЅЃЌБивЊЪБПЩМгШШ)ЃЌФмДяЕНЯргІЪЕбщФПЕФЕФЪЧ
бЁЯю | ЪдМС1 | ЪдМС2 | ЪдМС3 | ЪЕбщФПЕФ | зАжУ |
A | ХЈ | CuЦЌ | KI-ЕэЗлШмвК | бщжЄ |
|
B | бЮЫс | ЪЏЛвЪЏ | БЅКЭ | жЦБИ | |
C | ЯЁСђЫс | ШмвКX | ГЮЧхЪЏЛвЫЎ | бщжЄШмвКXжаЪЧЗёга | |
D | 70%СђЫс |
| Ысад | жЄУї |
A. A B. B C. C D. D
ЁОЬтФПЁПЙЄвЕЩЯдкКуШнУмБеШнЦїжагУЯТСаЗДгІКЯГЩМзДМЃКCOЃЈgЃЉ+2H2ЃЈgЃЉ![]() CH3OHЃЈgЃЉЁїH
CH3OHЃЈgЃЉЁїH
ЃЈ1ЃЉИУЗДгІЕФЦНКтГЃЪ§БэДяЪНЮЊ_______ЃЛ
ЃЈ2ЃЉШчБэЫљСаЪ§ОнЪЧЗДгІдкВЛЭЌЮТЖШЯТЕФЛЏбЇЦНКтГЃЪ§ЃЈKЃЉ
ЮТЖШ | 250Ёц | 300Ёц | 350Ёц |
K | 2.041 | 0.270 | 0.012 |
ЂйгЩБэжаЪ§ОнХаЖЯИУЗДгІЕФЁїH______0ЃЈЬюЁАЃОЁБЁЂЁА=ЁБЛђЁАЃМЁБЃЉЃЛ
ЂкФГЮТЖШЯТЃЌНЋ2molCOКЭ6molH2ГфШы2LЕФУмБеШнЦїжаЃЌГфЗжЗДгІ10sКѓДяЕНЦНКтЪБВтЕУcЃЈCOЃЉ=0.2mol/LЃЌдђCOЕФзЊЛЏТЪЮЊ____ЃЌгУH2БэЪОЗДгІЫйТЪЮЊ_____ЃЌДЫЪБЕФЮТЖШЮЊ______ЃЛ
ЃЈ3ЃЉвЊЬсИпCOЕФзЊЛЏТЪЃЌПЩвдВЩШЁЕФДыЪЉЪЧ______ЃЛ
aЃЎЩ§ЮТ bЃЎМгШыДпЛЏМС cЃЎдіМгCOЕФХЈЖШ
dЃЎКуШнГфШыH2 eЃЎКубЙГфШыЖшадЦјЬх fЃЎЗжРыГіМзДМ