��Ŀ����
14��������H��������·�ߺϳɣ�
��֪��R-CH=CH2$��_{��ii��H_{2}O_{2}/OH}^{��i��B_{2}H_{6}}$RCH2CH2OH
��ش��������⣺
��1����״����11.2L��̬��A�������г��ȼ�տ�������88g CO2��45g H2O����A���ӽṹ����3��������A�Ľṹ��ʽΪCH3CH��CH3��CH3��
��2��B��C��Ϊһ�ȴ�����D�����ƣ�ϵͳ������Ϊ2-����ϩ��
��3���ڴ���������1mol F��2mol H2��Ӧ������3-����-1-������F�Ľṹ��ʽ��
 ��д��E��G��Ӧ����H�Ľṹ��ʽ��
��д��E��G��Ӧ����H�Ľṹ��ʽ�� ��
����4����Ӧ�ٵķ�Ӧ��������ȥ��Ӧ��
��5������д��F������Cu��OH��2����Һ��Ӧ�Ļ�ѧ����ʽ
 +2Cu��OH��2$\stackrel{��}{��}$
+2Cu��OH��2$\stackrel{��}{��}$ +Cu2O��+2H2O��
+Cu2O��+2H2O����6����G������ͬ�����ŵķ�����ͬ���칹����4�֣�
���� 88gCO2�����ʵ���Ϊ��$\frac{88g}{44g/mol}$=2mol��45gH2O�����ʵ���Ϊ��$\frac{45g}{18g/mol}$=2.5mol�������11.2L��AΪ�����ʵ���Ϊ��$\frac{11.2L}{22.4L/mol}$=0.5mol��������A�к�̼ԭ��Ϊ4��Hԭ����Ϊ10����A��ѧʽΪC4H10��A�к���3��������AΪ�춡�飺CH3CH��CH3��CH3��A��Cl2���շ���ȡ����Ӧ��B��C��Ϊһ�ȴ�������B��CΪ2-��-1-�ȱ����2-��-2-�ȱ��飻B��C������ȥ��Ӧ����D����DΪCH2=C��CH3��2��D������Ϣ�еķ�Ӧ����E����EΪ��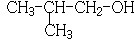 ��F������Cu��OH��2��Ӧ����ӦΪȩ������H2֮��Ϊ1��2�ӳɣ���Ӧ����̼̼˫���������ɵIJ���3-����-1-����������F�Ľṹ��ʽΪ��
��F������Cu��OH��2��Ӧ����ӦΪȩ������H2֮��Ϊ1��2�ӳɣ���Ӧ����̼̼˫���������ɵIJ���3-����-1-����������F�Ľṹ��ʽΪ��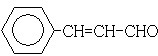 ��F��ȩ������Ϊ�Ȼ�����G����GΪ��
��F��ȩ������Ϊ�Ȼ�����G����GΪ�� ��D��E����������Ӧ����H����HΪ��
��D��E����������Ӧ����H����HΪ�� ���ݴ˽��н��
���ݴ˽��н��
��� �⣺88gCO2�����ʵ���Ϊ��$\frac{88g}{44g/mol}$=2mol��45gH2O�����ʵ���Ϊ��$\frac{45g}{18g/mol}$=2.5mol�������11.2L��AΪ�����ʵ���Ϊ��$\frac{11.2L}{22.4L/mol}$=0.5mol��������A�к�̼ԭ��Ϊ4��Hԭ����Ϊ10����A��ѧʽΪC4H10��A�к���3��������AΪ�춡�飺CH3CH��CH3��CH3��A��Cl2���շ���ȡ����Ӧ��B��C��Ϊһ�ȴ�������B��CΪ2-��-1-�ȱ����2-��-2-�ȱ��飻B��C������ȥ��Ӧ����D����DΪCH2=C��CH3��2��D������Ϣ�еķ�Ӧ����E����EΪ��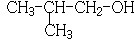 ��F������Cu��OH��2��Ӧ����ӦΪȩ������H2֮��Ϊ1��2�ӳɣ���Ӧ����̼̼˫���������ɵIJ���3-����-1-����������F�Ľṹ��ʽΪ��
��F������Cu��OH��2��Ӧ����ӦΪȩ������H2֮��Ϊ1��2�ӳɣ���Ӧ����̼̼˫���������ɵIJ���3-����-1-����������F�Ľṹ��ʽΪ��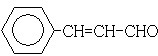 ��F��ȩ������Ϊ�Ȼ�����G����GΪ��
��F��ȩ������Ϊ�Ȼ�����G����GΪ�� ��D��E����������Ӧ����H��
��D��E����������Ӧ����H�� ����HΪ��
����H�� ��
��
��1�����ݷ�����֪��A�Ľṹ��ʽΪ��CH3CH��CH3��CH3���ʴ�Ϊ��CH3CH��CH3��CH3��
��2��D�Ľṹ��ʽΪ��CH2=C��CH3��2��Ϊϩ����̼̼˫����1��C������2��C�����л�������Ϊ��2-����ϩ��
�ʴ�Ϊ��2-����ϩ��
��3��F������Cu��OH��2��Ӧ����ӦΪȩ������H2֮��Ϊ1��2�ӳɣ���Ӧ����̼̼˫���������ɵIJ���3-����-1-����������F�Ľṹ��ʽΪ 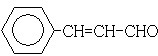 ��E��G��Ũ���������¿��Է���������Ӧ��
��E��G��Ũ���������¿��Է���������Ӧ�� ����H�Ľṹ��ʽΪ��
����H�Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
�� ��
��
��4����Ӧ��Ϊ±�����ڴ���Һ�е���ȥ��Ӧ���ʴ�Ϊ����ȥ��Ӧ��
��5��F�����Ƶ�Cu��OH��2����������G�� ����Ӧ����ʽΪ��
����Ӧ����ʽΪ�� +2Cu��OH��2$\stackrel{��}{��}$
+2Cu��OH��2$\stackrel{��}{��}$ +Cu2O��+2H2O��
+Cu2O��+2H2O��
�ʴ�Ϊ�� +2Cu��OH��2$\stackrel{��}{��}$
+2Cu��OH��2$\stackrel{��}{��}$ +Cu2O��+2H2O��
+Cu2O��+2H2O��
��6��GΪ ����G������ͬ�����ŵķ�����ͬ���칹�壬˵����ͬ���칹���к���̼̼˫�����Ȼ��ͱ��������Խ�����������Ӧ��λ�ñ任���ó��䷼�����ͬ���칹�壮������ͬ���칹���У�
����G������ͬ�����ŵķ�����ͬ���칹�壬˵����ͬ���칹���к���̼̼˫�����Ȼ��ͱ��������Խ�����������Ӧ��λ�ñ任���ó��䷼�����ͬ���칹�壮������ͬ���칹���У� ��
��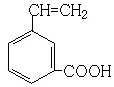 ��
�� ��
��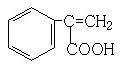 ���ܹ���4�֣�
���ܹ���4�֣�
�ʴ�Ϊ��4��
���� ���⿼���л����ƶϣ���Ŀ�Ѷ��еȣ�������ؿ���ѧ���������ƶϡ�֪ʶǨ����������ȷ�ƶ�A�Ľṹ�ǽⱾ��ؼ����������ʹ�����ȷ�����ʣ�ע���������Ϣ���з������ѵ���ͬ���칹��������жϣ�

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д���Ԫ������ ��ԭ������ �۷������� �����ʵ����� �����ʵ������� �����ʵ���������
| A�� | �٢ڢۢ� | B�� | �٢ڢ� | C�� | �ڢݢ� | D�� | �٢ܢݢ� |
| A�� | �ﵽƽ��ʱ��SO2��Ũ����SO3��Ũ����� | |
| B�� | ʹ�ô�����Ϊ�˼ӿ췴Ӧ���ʣ��������Ч�� | |
| C�� | Ϊ�����SO2��ת���ʣ�Ӧ�ʵ����O2��Ũ�� | |
| D�� | �����������£�SO2������100%ת��ΪSO3 |
| A�� | Cr | B�� | Mg | C�� | Fe | D�� | Ti |
| A�� | �������л��߷��� | B�� | ���ܲ�������ɫ��Ⱦ�� | ||
| C�� | ���γɵĵ�������ϩ | D�� | ����ϩ�к���˫�����ȶ����ױ��� |

 ��д�ṹ��ʽ����
��д�ṹ��ʽ���� ��һ�������¿�ˮ��Ϊ
��һ�������¿�ˮ��Ϊ ��R1-NH2����F��ǿ��ͳ�ʱ����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ��
��R1-NH2����F��ǿ��ͳ�ʱ����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ�� ��
�� ���۽��ʯ���ܰ�ˮ����CH3CH2CH2CH3����${\;}_{17}^{37}$Cl����${\;}_{17}^{35}$Cl����C70���ᰱ����
���۽��ʯ���ܰ�ˮ����CH3CH2CH2CH3����${\;}_{17}^{37}$Cl����${\;}_{17}^{35}$Cl����C70���ᰱ����