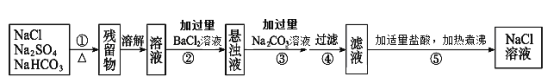
��Ŀ����
����Ŀ��ͭ����Ҫ�Ľ������㷺Ӧ���ڵ�������е���졢����������ͭ�Ļ������ڿ�ѧ�о���ũҵ��������������;���ش��������⣺
(1)CuSO4������Sԭ�ӵ��ӻ���ʽΪ________��SO42-�����幹��Ϊ_______________��
(2)��ϸͭ�ۿ�����������ϡ������ȣ����Ʊ��������£�
![]()
��NH4CuSO3�н��������ӵĺ�������Ų�ʽΪ__________________��N��O��S����Ԫ�صĵ�һ�����ܴ�С˳��Ϊ__________________________(��Ԫ�ط���)��
����CuSO4��Һ�м��������ˮ��������[Cu(NH3)4]SO4������˵����ȷ����________
A��������������ˮ��ԭ��֮һ��NH3���Ӻ�H2O����֮���γ������Ե��
B��NH3���Ӻ�H2O���ӣ����ӿռ乹�Ͳ�ͬ���������ӵļ���С��ˮ���ӵļ���
C��Cu(NH3)4]SO4��Һ�м����Ҵ�������������ɫ�ľ���
D����֪3.4 g��������������ȫȼ����������Ⱦ�����壬���ų�a kJ��������NH3��ȼ���ȵ��Ȼ�ѧ����ʽΪ��NH3(g)��3/4O2(g)=1/2N2(g)��3/2H2O(g) ��H��-5a kJ��mol��1
(3)����ͭ��Һ�е��백��������(H2NCH2COONa)���ɵõ������A����ṹ������ͼ��ʾ��


��1 mol����������(H2NCH2COONa)���ЦҼ�����ĿΪ________________��
�ڰ��������Ʒֽ����֮һΪ������̼��д��������̼��һ�ֵȵ����壺____________(д��ѧʽ)��
����֪������ͭ���տ�������һ�ֺ�ɫ���壬��ṹ������ͼ��ʾ����û�����Ļ�ѧʽ��________________��
���𰸡� sp3 �������� 1s22s22p63s23p63d10(��[Ar]3d10) N��O��S AC 8��6.02��1023 N2O(��SCN����NO3-��) Cu2O
��������(1)CuSO4������Sԭ�ӵļ۲���Ӷ�����![]() ��4���µ��Ӷ���Ϊ0����ȡsp3�ӻ���SO42-�����幹��Ϊ���������Σ�(2) ��NH4CuSO3�е�����������Cu+�����ĺ�������Ų��ǣ�1s22s22p63s23p63d10(��[Ar]3d10)������ͬһ���ڵ�һ�����ܱ仯���ɼ��ڢ�A����A����֪����һ�����ܴ�С˳��Ϊ��N>O>S����A��������������ˮ��ԭ��֮һ��NH3���Ӻ�H2O����֮���γ������Ե�ʣ�ѡ��A��ȷ��NH3���Ӻ�H2O���ӣ����ӿռ乹�Ͳ�ͬ�������������ͣ�ˮ��V�Σ��������ӵļ��Ǵ���ˮ���ӵļ��ǣ�ѡ��B����C��Cu(NH3)4]SO4��Һ�м����Ҵ�������������ɫ�ľ��壬ѡ��C��ȷ��D��ȼ���ȱ����������ȶ����������Ӧ�в���������ˮ�����������ȶ��������ѡ��D����ѡAC����3���ٰ��������ƽṹ�к���N-H 2����C-H 2����̼��������˫����һ����N-C��C-C��һ����8���Ҽ����ڵȵ�����Ϊ�۵�������ԭ�Ӹ�����ͬ���ʲ������»��������ҵ��ȷ�����дΪN2O��SCN����N3���ȣ��۸��ݾ�̯�����������Ϊ8��
��4���µ��Ӷ���Ϊ0����ȡsp3�ӻ���SO42-�����幹��Ϊ���������Σ�(2) ��NH4CuSO3�е�����������Cu+�����ĺ�������Ų��ǣ�1s22s22p63s23p63d10(��[Ar]3d10)������ͬһ���ڵ�һ�����ܱ仯���ɼ��ڢ�A����A����֪����һ�����ܴ�С˳��Ϊ��N>O>S����A��������������ˮ��ԭ��֮һ��NH3���Ӻ�H2O����֮���γ������Ե�ʣ�ѡ��A��ȷ��NH3���Ӻ�H2O���ӣ����ӿռ乹�Ͳ�ͬ�������������ͣ�ˮ��V�Σ��������ӵļ��Ǵ���ˮ���ӵļ��ǣ�ѡ��B����C��Cu(NH3)4]SO4��Һ�м����Ҵ�������������ɫ�ľ��壬ѡ��C��ȷ��D��ȼ���ȱ����������ȶ����������Ӧ�в���������ˮ�����������ȶ��������ѡ��D����ѡAC����3���ٰ��������ƽṹ�к���N-H 2����C-H 2����̼��������˫����һ����N-C��C-C��һ����8���Ҽ����ڵȵ�����Ϊ�۵�������ԭ�Ӹ�����ͬ���ʲ������»��������ҵ��ȷ�����дΪN2O��SCN����N3���ȣ��۸��ݾ�̯�����������Ϊ8��![]() ��1=2 ����Ϊ4����ȡ�������ȵû�ѧʽΪCu2O��
��1=2 ����Ϊ4����ȡ�������ȵû�ѧʽΪCu2O��

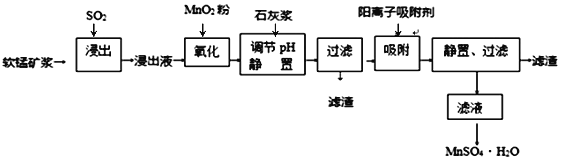
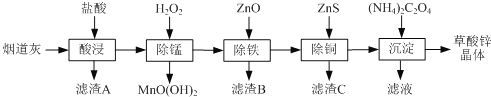
����Ŀ������п�����̵���(��Ҫ�ɷ�ΪZnO����������Fe2O3��CuO��SiO2��MnO��)Ϊԭ�Ͽ���������п����(ZnC2O4��2H2O)��
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
�������� | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
��ʼ������pH | 1.5 | 6.5 | 4.2 | 5.4 |
������ȫ��pH | 3.3 | 9.7 | 6.7 | 8.2 |
���ʴ��������⣺
��1������B����Ҫ�ɷ�Ϊ________ ��
��2�����̹����в���MnO(OH)2���������ӷ���ʽΪ________��
��3���ٳ���(����Cu2�����ܱ���ȥ)ʱ����ZnO���Ʒ�ӦҺpH�ķ�ΧΪ___________��
�����������г������ͭ��˳���ܵߵ�����������ʻ��С����ԭ����________��
��4�����������̲���Na2C2O4�������茶�����������п�������ļ��Ϸ�ʽ��________��
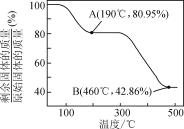
��5��������п������ȷֽ�ɵõ�һ�����ײ��ϡ����ȹ����й�����������¶ȵı仯��ͼ��ʾ��300 �桫460 �淶Χ�ڣ�������Ӧ�Ļ�ѧ����ʽΪ________��
����Ŀ�������±���ʾ��ѧ��Ӧ�����ݹ�ϵ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶� | |
973 K | 1 173 K | ||
��Fe(s)��CO2(g) | K1 | 1.47 | 2.15 |
��Fe(s)��H2O(g) | K2 | 2.38 | 1.67 |
��CO(g)��H2O(g) | K3 | �� | �� |
��ش�
(1)��Ӧ����________(��������������������)��Ӧ��
(2)д����Ӧ�۵�ƽ�ⳣ��K3�ı���ʽ_______________________��
(3)���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3��________(��K1��K2��ʾ)��
(4)Ҫʹ��Ӧ����һ�������½�����ƽ�����淴Ӧ�����ƶ����ɲ�ȡ�Ĵ�ʩ��________(��д��ĸ���)��
A����С��Ӧ�������ݻ� B������Ӧ�������ݻ�
C�������¶� D��ʹ�ú��ʵĴ���
E���跨��Сƽ����ϵ�е�COŨ��
(5)����Ӧ�۵��淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
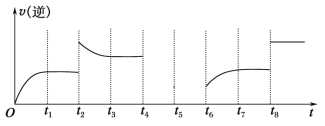
�ٿɼ���Ӧ��t1��t3��t7ʱ���ﵽ��ƽ�⣬��t2��t8ʱ���ı���һ�����������жϸı����ʲô������t2ʱ________��t8ʱ________��
����t4ʱ��ѹ��t6ʱ����Ӧ���Ũ�ȣ�����ͼ�л���t4��t6ʱ�淴Ӧ������ʱ��Ĺ�ϵ����__________��