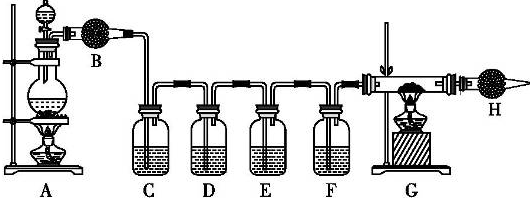
��Ŀ����
6����֪����̼��������Ԫ�ص�ijЩ���ʾ������ƵĽṹ�����ʣ���ش��������⣺��1����Ԫ�ص��⻯���H2O�⣬����H2O2��̼Ԫ�ص��⻯���CH4�⣬����C2H6�ȣ���Ԫ�ص��⻯���NH3�⣬���к�2����ԭ�ӵķ��ӵĻ�ѧʽΪN2H4����е�Ȱ����ߣ���ߡ��͡��������⻯�����������ᷴӦ�Ļ�ѧ��Ӧ����ʽΪ��N2H4+2HCl=N2H6Cl2��
��2��������̼ԭ�ӣ���ԭ�Ӽ�Ҳ���γ���״�ṹ��ij��״�ṹ�ĵ��⻯�����У���ԭ�Ӽ�ֻ��N-N������ʽ�����γ��⻯���ϵ�е��⻯���ﻯѧͨʽΪNnH n+2��n��1��nΪ����������n��ʾ��ԭ�Ӹ�������
��3�������й㷺ʹ���������ʣ�CmHn��ȼ�ϣ��������ʱ�ĸ���ȼ�ϳ��õ��⻯���������N2H4��2014��10��31���ڲ��Է���ʱ�ٵ�ά�����ӹ�˾��̫�մ�2�š���ҵ���˷ɴ���N2H4ȼ�ϣ�����ȼ��ѡ����˵���¹�ԭ��֮һ������������ȼ�������ɵ�����ԭ����ɣ�������ԭ����������������CO2������ԭ����������������ͬ������ȼ���Ļ�ѧʽΪN2O������ȼ�շ�Ӧ������ɫ��������д����ȼ�շ�Ӧ�Ļ�ѧ����ʽΪN2H4+2N2O=3N2+2H2O
��4������ʽΪN4�ĵ�Ԫ�ص�ͬ�������壬�ṹ��ͬ�ڰ��ף�N4��ÿ��ԭ�����������8e-�ṹ����1molN4�к���6NA�Թ��õ��Ӷԣ���֪NH3��-NH2��
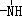 ��
�� һ�������¶����Ժ�H+��ϣ���N4��������H+��Ӧ���ɵ����ӵĻ�ѧʽΪN4H44+��
һ�������¶����Ժ�H+��ϣ���N4��������H+��Ӧ���ɵ����ӵĻ�ѧʽΪN4H44+����5����ѧ�ҷ�����һ�ֻ�ѧʽΪN4H4�����ӻ����һ��������1mol N4H4���ڵ��������������ӣ�����һ��ΪNH4+������������ʱ���뷽��ʽΪN4H4=NH4++N3-��
���� ��1��������Ϣ��ϳ�ʶ�жϺ�2����ԭ�ӵķ���Ϊ�£�������Է���������С�ж����۷е㣬Ȼ����д��ѧ��Ӧ����ʽ���ɣ�
��2��ÿ��Nԭ�����γ�3�����ۼ������Ժ͵�ԭ�ӡ���ԭ�Ӷ����γɹ��ۼ���������ѧ���ɷ�ȷ����ͨʽ��
��3�����ݸ�N��O�������������̼���ڵȵ����壬�ƶϳ��������T�ɣ���ɫ����������ȼ�ղ���Ϊ�����������⣬�ݴ���д���ɣ�
��4��������Ϊ�������壬ÿ���߶�����һ��P-P�������Թ�6��P-P���ۼ����ݴ��ƶϼ��ɣ�N4��ÿ��ԭ�����������8e-�ṹ��������1�Թ¶Ե��ӣ�ÿ��Nԭ�ӻ��ܵ������1��H���ӣ��ݴ˽�ɣ�
��5���������⣬��ϵ�Ԫ�ء�HԪ���غ���д���뷽��ʽ���ɣ�
��� �⣺��1����2����ԭ�ӵķ���Ϊ�£���ѧʽΪN2H4����������Է��������ϰ������۷е�Ȱ����ߣ�����HCl��Ӧ����ʽΪN2H4+2HCl=N2H6Cl2��
�ʴ�Ϊ��N2H4���ߣ�N2H4+2HCl=N2H6Cl2��
��2������ԭ�Ӹ���Ϊ1ʱ����ԭ�Ӹ���Ϊ3��
����ԭ�Ӹ���Ϊ2ʱ����ԭ�Ӹ���Ϊ4��
����ԭ�Ӹ���Ϊ3ʱ����ԭ�Ӹ���Ϊ5��
����ԭ�Ӹ���Ϊ4ʱ����ԭ�Ӹ���Ϊ6��
����ԭ�Ӹ���Ϊnʱ����ԭ�Ӹ���Ϊn+2��
������ͨʽΪNnH n+2��n��1��nΪ����������
�ʴ�Ϊ��NnH n+2��n��1��nΪ����������
��3������ȼ�������ɵ�����ԭ����ɣ�������ԭ����������������CO2������ԭ����������������ͬ���ʸ���ȼ��Ϊ��N2O�����ڸ÷�Ӧ������ɫ��������N�IJ���ӦΪ����������������ԭ��Ӧԭ����д����ѧ��Ӧ����ʽΪ��N2H4+2N2O=3N2+2H2O��
�ʴ�Ϊ��N2O��N2H4+2N2O=3N2+2H2O��
��4��������Ϊ�������壬ÿ���߶�����һ��P-P�������Թ�6��P-P���ۼ���N4�Ľṹ��ͬ�ڰ��ף���1molN4�к���6molN-N����N4��ÿ��ԭ�����������8e-�ṹ��������1�Թ¶Ե��ӣ�ÿ��Nԭ�ӻ����ṩ1���չ�������1��H���ӣ��ʿ����γ�N4H44+��
�ʴ�Ϊ��6NA��N4H44+��
��5��1mol N4H4���ڵ��������������ӣ�����һ��ΪNH4+������NԪ����HԪ���غ��֪����һ������ӦΪ��N3-�����뷽��ʽΪ��N4H4=NH4++N3-��
�ʴ�Ϊ��N4H4=NH4++N3-��
���� ������Ҫ������ǵ�Ԫ�ؼ��仯����漰���Ļ���������ʡ��۷е�ıȽϣ�����ʽ����д�����ۼ��ȣ������֪ʶ��϶࣬�Ѷ��еȣ������ڿ���ѧ���Ի���֪ʶ���ۺ�Ӧ��������

 ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�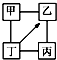 ͼ�С�-����ʾ���������ʼ���һ�������¿��Է�Ӧ����-������ʾ����һ�������¿���ת��Ϊ�ң���������ѡ���У�����ͼʾҪ����ǣ�������
ͼ�С�-����ʾ���������ʼ���һ�������¿��Է�Ӧ����-������ʾ����һ�������¿���ת��Ϊ�ң���������ѡ���У�����ͼʾҪ����ǣ�������| �� | �� | �� | �� | |
| A | H2SO4 | Na2SO4 | NaOH | NaCl |
| B | KCl | K2CO3 | KOH | HCl |
| C | O2 | CO | CuO | C |
| D | Fe | CuCl2 | Zn | HCl |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ͬһ����Ԫ�أ�ԭ�Ӱ뾶Խ���䵥�ʵ��۵㲻һ��Խ�� | |
| B�� | ȫ���ɷǽ���Ԫ����ɵĻ�������һ��ֻ�й��ۼ� | |
| C�� | ����пƬ��ͭƬ��ϡ������ɵ�ԭ����У�������ͭƬ�����·����пƬ | |
| D�� | �Ȼ��ƺ��Ȼ���ֱ��ܽ���ˮ�����˷������Ӽ����������ͬ������ |
| A�� | NaHA��Һһ�������� | B�� | c��H+��•c��OH-��=1��10-14x | ||
| C�� | c��Na+��=c��K+�� | D�� | c��Na+��+c��K+��=0.05 mol/L |
| A�� | ��ϡ�����м���ͭ�ۣ�ͭ�۲��ܽ⣬�ټ���Cu��NO3��2���壬ͭ���Բ��ܽ� | |
| B�� | ij������ʹʪ��ĺ�ɫʯ����ֽ�������������ˮ��Һһ���Լ��� | |
| C�� | ͭ��ϡ���ᷴӦ�����ӷ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O | |
| D�� | HNO3��NO��NO2�����ϸ����仯����ͨ��һ��ʵ�� |
| A�� | ����һ�����ʵ���Ũ�ȵ���Һ������ʱ���ӿ̶��� | |
| B�� | ����һ���������������ҩƷ�������̣�����������̣������ƶ�����ʹ֮ƽ�� | |
| C�� | �к͵ζ��õ���ƿ�������Һ���ټ���������ˮϡ�� | |
| D�� | �ڳ��������в����������ƺ�ϡ���ᷴӦ���к��� |
| A�� | ϡ���ᡢϡ������ܽ�ľ̿�����ɶ�����̼ | |
| B�� | Na2O2��ˮ��Ӧ�����ȵ�Fe��ˮ������Ӧ������������ | |
| C�� | Li��C��P�ֱ�������������ȼ�վ�����һ����Ӧ������ | |
| D�� | NaHCO3��Na2CO3����NH4��2CO3���ֹ������Ⱥ������������ |
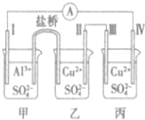 ij�绯ѧװ����ͼ��ʾ���缫IΪAl��������ΪCu���ҿ�ʼʱ�ĵ缫��������ȣ�����������ȷ���ǣ�������
ij�绯ѧװ����ͼ��ʾ���缫IΪAl��������ΪCu���ҿ�ʼʱ�ĵ缫��������ȣ�����������ȷ���ǣ�������| A�� | ��Ϊԭ��أ��ҡ�����Ϊ���� | |
| B�� | �����������缫����A���缫I | |
| C�� | ��0.1mol����ת��ʱ���缫I�͵缫����������Ϊ4.1g | |
| D�� | �����еĵ���ʸ�ΪCuCl2���缫��ĵ缫��Ӧ�����ı� |