��Ŀ����
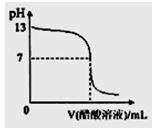
�����£���0.1000mol��L-1NaOH��Һ�ζ�20.00mL 0.1000mol��L-1CH3COOH��Һ���ζ���������ͼ������˵����ȷ����
��H+�ݣ���Na+������OH����

| A�������ʾ��Һ�У���CH3COO-��+��OH-��=��CH3COOH�� +��H+�� |
| B�������ʾ��Һ�У���Na+��=��CH3COOH��+��CH3COO-�� |
| C�������ʾ��Һ�У���Na+�ݣ���OH���ݣ���CH3COO-��>��H+�� |
| D���ζ������п��ܳ��֣���CH3COOH�ݣ���CH3COO-�ݣ� |
D
A ����Ӧ��ѭ����غ㣬��CH3COO-��+��OH-��=��Na+��+��H+��
B �������ʾ��ҺPH=7�������ԣ���Na+��=��CH3COO-��
C �������ʾ��Һ�У���Na+�ݣ���CH3COO-�ݣ���OH����>��H+��
D ��ȷ����CH3COOH��Һ�п�ʼ�����������NaOH��Һʱ���ִ��������
B �������ʾ��ҺPH=7�������ԣ���Na+��=��CH3COO-��
C �������ʾ��Һ�У���Na+�ݣ���CH3COO-�ݣ���OH����>��H+��
D ��ȷ����CH3COOH��Һ�п�ʼ�����������NaOH��Һʱ���ִ��������

��ϰ��ϵ�д�
�����Ŀ
 ijһԪ��
ijһԪ�� ��Һ��
��Һ��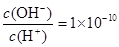 ������˵����ȷ����( )
������˵����ȷ����( )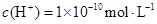
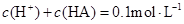
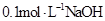 ��Һ�������Ϻ�������Һ��
��Һ�������Ϻ�������Һ��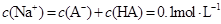
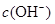 ������
������